Three-component coupling sequence for the regiospecific synthesis of substituted pyridines
- PMID: 22103772
- PMCID: PMC3262091
- DOI: 10.1021/ja2105703
Three-component coupling sequence for the regiospecific synthesis of substituted pyridines
Abstract
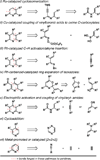
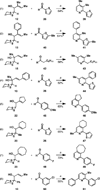
A de novo synthesis of substituted pyridines is described that proceeds through nucleophilic addition of a dithiane anion to an α,β-unsaturated carbonyl followed by metallacycle-mediated union of the resulting allylic alcohol with preformed trimethylsilane-imines (generated in situ by the low-temperature reaction of lithium hexamethyldisilazide with an aldehyde) and Ag(I)- or Hg(II)-mediated ring closure. The process is useful for the convergent assembly of di- through penta-substituted pyridines with complete regiochemical control.
© 2011 American Chemical Society
Figures







References
-
-
For a recent review, see: Henry GD. Tetrahedron. 2004;60:6043–6061.
-
-
-
Name Reactions in Heterocycle Synthesis; Li JJ, Corey EJ, editors. New York: Wiley; 2005. p. 558.
-
-
- Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:4592–4593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

