The Nef-infectivity enigma: mechanisms of enhanced lentiviral infection
- PMID: 22103831
- PMCID: PMC3355465
- DOI: 10.2174/157016211798842099
The Nef-infectivity enigma: mechanisms of enhanced lentiviral infection
Abstract
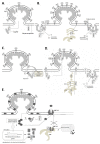
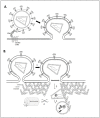
The Nef protein is an essential factor for lentiviral pathogenesis in humans and other simians. Despite a multitude of functions attributed to this protein, the exact role of Nef in disease progression remains unclear. One of its most intriguing functions is the ability of Nef to enhance the infectivity of viral particles. In this review we will discuss current insights in the mechanism of this well-known, yet poorly understood Nef effect. We will elaborate on effects of Nef, on both virion biogenesis and the early stage of the cellular infection, that might be involved in infectivity enhancement. In addition, we provide an overview of different HIV-1 Nef domains important for optimal infectivity and briefly discuss some possible sources of the frequent discrepancies in the field. Hereby we aim to contribute to a better understanding of this highly conserved and therapeutically attractive Nef function.
Figures
References
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2010. Available from: http://www.unaids.org/documents /20101123_GlobalReport_em.pdf . 2010. [cited 2011 11th June].
-
- Allers K, Hutter G, Hofmann J, et al. Evidence for the cure of HIV infection by CCR5Delta32/Delta32 stem cell transplantation. Blood. 2011;117:2791–9. - PubMed
-
- Richter SN, Frasson I, Palu G. Strategies for inhibiting function of HIV-1 accessory proteins: a necessary route to AIDS therapy? Curr Med Chem. 2009;16:267–86. - PubMed
-
- Malim MH, Emerman M. HIV-1 accessory proteins--ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
