Resin-based investigation of acyl carrier protein interaction networks in Escherichia coli
- PMID: 22104437
- PMCID: PMC3809020
- DOI: 10.1016/j.bmc.2011.10.053
Resin-based investigation of acyl carrier protein interaction networks in Escherichia coli
Abstract
Protein-protein interactions play an integral role in metabolic regulation. Elucidation of these networks is complicated by the changing identity of the proteins themselves. Here we demonstrate a resin-based technique that leverages the unique tools for acyl carrier protein (ACP) modification with non-hydrolyzable linkages. ACPs from Escherichia coli and Shewanella oneidensis MR-1 are bound to Affigel-15 with varying acyl groups attached and introduced to proteomic samples. Isolation of these binding partners is followed by MudPIT analysis to identify each interactome with the variable of ACP-tethered substrates. These techniques allow for investigation of protein interaction networks with the changing identity of a given protein target.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
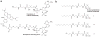


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

