Inhibition and dispersal of Agrobacterium tumefaciens biofilms by a small diffusible Pseudomonas aeruginosa exoproduct(s)
- PMID: 22105093
- PMCID: PMC3703781
- DOI: 10.1007/s00203-011-0767-9
Inhibition and dispersal of Agrobacterium tumefaciens biofilms by a small diffusible Pseudomonas aeruginosa exoproduct(s)
Abstract
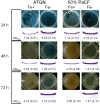
Environmental biofilms often contain mixed populations of different species. In these dense communities, competition between biofilm residents for limited nutrients such as iron can be fierce, leading to the evolution of competitive factors that affect the ability of competitors to grow or form biofilms. We have discovered a compound(s) present in the conditioned culture fluids of Pseudomonas aeruginosa that disperses and inhibits the formation of biofilms produced by the facultative plant pathogen Agrobacterium tumefaciens. The inhibitory activity is strongly induced when P. aeruginosa is cultivated in iron-limited conditions, but it does not function through iron sequestration. In addition, the production of the biofilm inhibitory activity is not regulated by the global iron regulatory protein Fur, the iron-responsive extracytoplasmic function σ factor PvdS, or three of the recognized P. aeruginosa quorum-sensing systems. In addition, the compound(s) responsible for the inhibition and dispersal of A. tumefaciens biofilm formation is likely distinct from the recently identified P. aeruginosa dispersal factor, cis-2-decenoic acid (CDA), as dialysis of the culture fluids showed that the inhibitory compound was larger than CDA and culture fluids that dispersed and inhibited biofilm formation by A. tumefaciens had no effect on biofilm formation by P. aeruginosa.
Figures


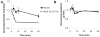

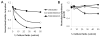

Similar articles
-
Quorum sensing and motility mediate interactions between Pseudomonas aeruginosa and Agrobacterium tumefaciens in biofilm cocultures.Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3828-33. doi: 10.1073/pnas.0511323103. Epub 2006 Feb 28. Proc Natl Acad Sci U S A. 2006. PMID: 16537456 Free PMC article.
-
Linoleic acid inhibits Pseudomonas aeruginosa biofilm formation by activating diffusible signal factor-mediated quorum sensing.Biotechnol Bioeng. 2021 Jan;118(1):82-93. doi: 10.1002/bit.27552. Epub 2020 Sep 15. Biotechnol Bioeng. 2021. PMID: 32880907
-
Proteomic analysis reveals modulation of iron homeostasis and oxidative stress response in Pseudomonas aeruginosa PAO1 by curcumin inhibiting quorum sensing regulated virulence factors and biofilm production.J Proteomics. 2016 Aug 11;145:112-126. doi: 10.1016/j.jprot.2016.04.019. Epub 2016 Apr 19. J Proteomics. 2016. PMID: 27108548
-
[Iron uptake and biofilm formation in Pseudomonas aeruginosa].Sheng Wu Gong Cheng Xue Bao. 2017 Sep 25;33(9):1489-1512. doi: 10.13345/j.cjb.170140. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956396 Review. Chinese.
-
Recent perspectives on the molecular basis of biofilm formation by Pseudomonas aeruginosa and approaches for treatment and biofilm dispersal.Folia Microbiol (Praha). 2018 Jul;63(4):413-432. doi: 10.1007/s12223-018-0585-4. Epub 2018 Jan 19. Folia Microbiol (Praha). 2018. PMID: 29352409 Review.
Cited by
-
Exploiting quorum sensing to confuse bacterial pathogens.Microbiol Mol Biol Rev. 2013 Mar;77(1):73-111. doi: 10.1128/MMBR.00046-12. Microbiol Mol Biol Rev. 2013. PMID: 23471618 Free PMC article. Review.
-
Regulation of flagellar motility during biofilm formation.FEMS Microbiol Rev. 2013 Nov;37(6):849-71. doi: 10.1111/1574-6976.12018. Epub 2013 Apr 12. FEMS Microbiol Rev. 2013. PMID: 23480406 Free PMC article. Review.
-
Agrobacterium tumefaciens deploys a superfamily of type VI secretion DNase effectors as weapons for interbacterial competition in planta.Cell Host Microbe. 2014 Jul 9;16(1):94-104. doi: 10.1016/j.chom.2014.06.002. Epub 2014 Jun 26. Cell Host Microbe. 2014. PMID: 24981331 Free PMC article.
-
Discrete Responses to Limitation for Iron and Manganese in Agrobacterium tumefaciens: Influence on Attachment and Biofilm Formation.J Bacteriol. 2015 Dec 28;198(5):816-29. doi: 10.1128/JB.00668-15. J Bacteriol. 2015. PMID: 26712936 Free PMC article.
-
Interbacterial Biofilm Competition through a Suite of Secreted Metabolites.ACS Chem Biol. 2024 Feb 16;19(2):462-470. doi: 10.1021/acschembio.3c00655. Epub 2024 Jan 23. ACS Chem Biol. 2024. PMID: 38261537 Free PMC article.
References
-
- Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. - PubMed
-
- Barton HA, Johnson Z, Cox CD, Vasil AI, Vasil ML. Ferric uptake regulator mutants of Pseudomonas aeruginosa with distinct alterations in the iron-dependent repression of exotoxin A and siderophores in aerobic and microaerobic environments. Mol Microbiol. 1996;21:1001–1017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

