Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice
- PMID: 22105170
- PMCID: PMC3226008
- DOI: 10.1172/JCI59777
Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice
Abstract
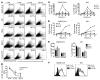
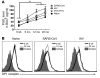
The morbidity and mortality associated with respiratory virus infection is felt most keenly among the elderly. T cells are necessary for viral clearance, and many age-dependent intrinsic T cell defects have been documented. However, the development of robust T cell responses in the lung also requires respiratory DCs (rDCs), which must process antigen and migrate to draining LNs (DLNs), and little is known about age-related defects in these T cell-extrinsic functions. Here, we show that increases in prostaglandin D(2) (PGD(2)) expression in mouse lungs upon aging correlate with a progressive impairment in rDC migration to DLNs. Decreased rDC migration resulted in diminished T cell responses and more severe clinical disease in older mice infected with respiratory viruses. Diminished rDC migration associated with virus-specific defects in T cell responses and was not a result of cell-intrinsic defect, rather it reflected the observed age-dependent increases in PGD(2) expression. Blocking PGD(2) function with small-molecule antagonists enhanced rDC migration, T cell responses, and survival. This effect correlated with upregulation on rDCs of CCR7, a chemokine receptor involved in DC chemotaxis. Our results suggest that inhibiting PGD(2) function may be a useful approach to enhance T cell responses against respiratory viruses in older humans.
Figures


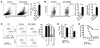
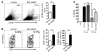
Comment in
-
Slowing down with age: lung DCs do it too.J Clin Invest. 2011 Dec;121(12):4636-9. doi: 10.1172/JCI61367. Epub 2011 Nov 21. J Clin Invest. 2011. PMID: 22105176 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

