Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells
- PMID: 22105488
- PMCID: PMC3271588
- DOI: 10.1038/cr.2011.181
Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells
Abstract
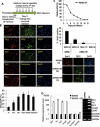
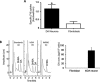
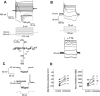
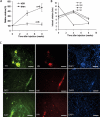
Transplantation of exogenous dopaminergic neuron (DA neurons) is a promising approach for treating Parkinson's disease (PD). However, a major stumbling block has been the lack of a reliable source of donor DA neurons. Here we show that a combination of five transcriptional factors Mash1, Ngn2, Sox2, Nurr1, and Pitx3 can directly and effectively reprogram human fibroblasts into DA neuron-like cells. The reprogrammed cells stained positive for various markers for DA neurons. They also showed characteristic DA uptake and production properties. Moreover, they exhibited DA neuron-specific electrophysiological profiles. Finally, they provided symptomatic relief in a rat PD model. Therefore, our directly reprogrammed DA neuron-like cells are a promising source of cell-replacement therapy for PD.
Figures
Similar articles
-
Reprogramming astrocytes into dopaminergic neurons to restore motor dysfunction in Parkinson's disease model rats.Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024 Sept 28;49(9):1377-1389. doi: 10.11817/j.issn.1672-7347.2024.240078. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024. PMID: 39931768 Free PMC article. Chinese, English.
-
Conditioned medium from human amniotic epithelial cells may induce the differentiation of human umbilical cord blood mesenchymal stem cells into dopaminergic neuron-like cells.J Neurosci Res. 2013 Jul;91(7):978-86. doi: 10.1002/jnr.23225. Epub 2013 Apr 30. J Neurosci Res. 2013. PMID: 23633297
-
ASCL1-mediated direct reprogramming: converting ventral midbrain astrocytes into dopaminergic neurons for Parkinson's disease therapy.BMB Rep. 2024 Aug;57(8):363-368. doi: 10.5483/BMBRep.2023-0222. BMB Rep. 2024. PMID: 38649147 Free PMC article.
-
The lifelong maintenance of mesencephalic dopaminergic neurons by Nurr1 and engrailed.J Biomed Sci. 2014 Apr 1;21(1):27. doi: 10.1186/1423-0127-21-27. J Biomed Sci. 2014. PMID: 24685177 Free PMC article. Review.
-
NURR1 in Parkinson disease--from pathogenesis to therapeutic potential.Nat Rev Neurol. 2013 Nov;9(11):629-36. doi: 10.1038/nrneurol.2013.209. Epub 2013 Oct 15. Nat Rev Neurol. 2013. PMID: 24126627 Review.
Cited by
-
Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons.Nat Commun. 2013;4:2183. doi: 10.1038/ncomms3183. Nat Commun. 2013. PMID: 23873306 Free PMC article.
-
Reactive astrocytes targeting with oral vitamin A: Efficient neuronal regeneration for Parkinson's disease treatment and reversal of associated liver fibrosis.CNS Neurosci Ther. 2023 Aug;29(8):2111-2128. doi: 10.1111/cns.14179. Epub 2023 Mar 22. CNS Neurosci Ther. 2023. PMID: 36949616 Free PMC article.
-
Development-inspired reprogramming of the mammalian central nervous system.Science. 2014 Jan 31;343(6170):1239882. doi: 10.1126/science.1239882. Science. 2014. PMID: 24482482 Free PMC article. Review.
-
Generation of integration-free induced hepatocyte-like cells from mouse fibroblasts.Sci Rep. 2015 Oct 27;5:15706. doi: 10.1038/srep15706. Sci Rep. 2015. PMID: 26503743 Free PMC article.
-
Cell Therapy for Parkinson's Disease: New Hope from Reprogramming Technologies.Aging Dis. 2015 Nov 17;6(6):499-503. doi: 10.14336/AD.2014.1201. eCollection 2015 Nov. Aging Dis. 2015. PMID: 26618051 Free PMC article. Review.
References
-
- Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–588. - PubMed
-
- Jenner P. Molecular mechanisms of L-DOPA-induced dyskinesia. Nat Rev Neurosci. 2008;9:665–677. - PubMed
-
- Brodsky MA, Nutt JG. Parkinson disease: deep brain stimulation versus best medical therapy for PD. Nat Rev Neurol. 2010;6:530–532. - PubMed
-
- Sladek JR, Jr, Redmond DE, Jr, Collier TJ, et al. Transplantation of fetal dopamine neurons in primate brain reverses MPTP induced parkinsonism. Prog Brain Res. 1987;71:309–323. - PubMed
-
- Lindvall O, Brundin P, Widner H, et al. Grafts of fetal dopamine neurons survive and improve motor function in Parkinson's disease. Science. 1990;247:574–577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA015050/DA/NIDA NIH HHS/United States
- NS065353/NS/NINDS NIH HHS/United States
- R01 CA155270/CA/NCI NIH HHS/United States
- CA136748/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- R01 CA136748/CA/NCI NIH HHS/United States
- R01 DA004216/DA/NIDA NIH HHS/United States
- CA155270/CA/NCI NIH HHS/United States
- R37 DA004216/DA/NIDA NIH HHS/United States
- K05 DA015050/DA/NIDA NIH HHS/United States
- DA004216/DA/NIDA NIH HHS/United States
- R01 CA131408/CA/NCI NIH HHS/United States
- R21 NS065353/NS/NINDS NIH HHS/United States
- CA131408/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

