Interferon regulatory factor-1 regulates the autophagic response in LPS-stimulated macrophages through nitric oxide
- PMID: 22105605
- PMCID: PMC3320143
- DOI: 10.2119/molmed.2011.00282
Interferon regulatory factor-1 regulates the autophagic response in LPS-stimulated macrophages through nitric oxide
Abstract
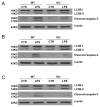
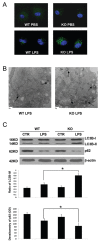
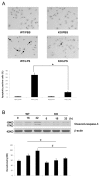
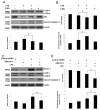
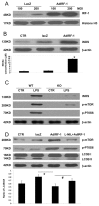
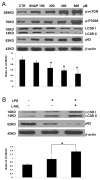
The pathogenesis of sepsis is complex and, unfortunately, poorly understood. The cellular process of autophagy is believed to play a protective role in sepsis; however, the mechanisms responsible for its regulation in this setting are ill defined. In the present study, interferon regulatory factor 1 (IRF-1) was found to regulate the autophagic response in lipopolysaccharide (LPS)-stimulated macrophages. In vivo, tissue macrophages obtained from LPS-stimulated IRF-1 knockout (KO) mice demonstrated increased autophagy and decreased apoptosis compared to those isolated from IRF-1 wild-type (WT) mice. In vitro, LPS-stimulated peritoneal macrophages obtained from IRF-1 KO mice experienced increased autophagy and decreased apoptosis. IRF-1 mediates the inhibition of autophagy by modulating the activation of the mammalian target of rapamycin (mTOR). LPS induced the activation of mTOR in WT peritoneal macrophages, but not in IRF-1 KO macrophages. In contrast, overexpression of IRF-1 alone increased the activation of mTOR and consequently decreased autophagic flux. Furthermore, the inhibitory effects of IRF-1 mTOR activity were mediated by nitric oxide (NO). Therefore, we propose a novel role for IRF-1 and NO in the regulation of macrophage autophagy during LPS stimulation in which IRF-1/NO inhibits autophagy through mTOR activation.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

