Selective deletion of the membrane-bound colony stimulating factor 1 isoform leads to high bone mass but does not protect against estrogen-deficiency bone loss
- PMID: 22105655
- PMCID: PMC4378684
- DOI: 10.1007/s00774-011-0336-y
Selective deletion of the membrane-bound colony stimulating factor 1 isoform leads to high bone mass but does not protect against estrogen-deficiency bone loss
Abstract
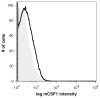
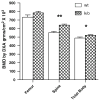
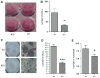
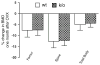
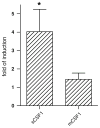
To better define the biologic function of membrane-bound CSF1 (mCSF1) in vivo, we have generated mCSF1 knockout (k/o) mice. Spinal bone density (BMD) was 15.9% higher in k/o mice compared to wild-type (wt) controls (P < 0.01) and total BMD was increased by 6.8% (P < 0.05). A higher mean femur BMD was also observed but did not reach statistical significance (6.9% P = NS). The osteoclastogenic potential of bone marrow isolated from mCSF1 k/o mice was reduced compared to wt marrow. There were no defects in osteoblast number or function suggesting that the basis for the high bone mass phenotype was reduced resorption. In addition to a skeletal phenotype, k/o mice had significantly elevated serum triglyceride levels (123 ± 7 vs. 88 ± 3.2 mg/dl; k/o vs. wt, P < 0.001), while serum cholesterol levels were similar (122 ± 6 vs. 116 ± 6 mg/dl; k/o vs. wt, P = NS). One month after surgery, 5-month-old k/o and wt female mice experienced the same degree of bone loss following ovariectomy (OVX). OVX induced a significant fourfold increase in the expression of the soluble CSF1 isoform (sCSF1) in the bones of wt mice while expression of mCSF1 was unchanged. These findings indicate that mCSF1 is essential for normal bone remodeling since, in its absence, BMD is increased. Membrane-bound CSF1 does not appear to be required for estrogen-deficiency bone loss while in contrast; our data suggest that sCSF1 could play a key role in this pathologic process. The reasons why mCSF1 k/o mice have hypertriglyceridemia are currently under study.
Figures

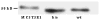
Similar articles
-
Selective deletion of the soluble Colony-Stimulating Factor 1 isoform in vivo prevents estrogen-deficiency bone loss in mice.Bone Res. 2017 Nov 14;5:17022. doi: 10.1038/boneres.2017.22. eCollection 2017. Bone Res. 2017. PMID: 29152381 Free PMC article.
-
Targeted overexpression of the two colony-stimulating factor-1 isoforms in osteoblasts differentially affects bone loss in ovariectomized mice.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E714-20. doi: 10.1152/ajpendo.90631.2008. Epub 2009 Jan 13. Am J Physiol Endocrinol Metab. 2009. PMID: 19141689 Free PMC article.
-
The cell-surface isoform of colony stimulating factor 1 (CSF1) restores but does not completely normalize fecundity in CSF1-deficient mice.Biol Reprod. 2006 Feb;74(2):331-6. doi: 10.1095/biolreprod.105.045047. Epub 2005 Oct 19. Biol Reprod. 2006. PMID: 16237150
-
Cellular and molecular effects of growth hormone and estrogen on human bone cells.APMIS Suppl. 1997;71:1-30. APMIS Suppl. 1997. PMID: 9357492 Review.
-
Interaction between bone and immune cells: Implications for postmenopausal osteoporosis.Semin Cell Dev Biol. 2022 Mar;123:14-21. doi: 10.1016/j.semcdb.2021.05.014. Epub 2021 May 20. Semin Cell Dev Biol. 2022. PMID: 34024716 Review.
Cited by
-
Selective deletion of the soluble Colony-Stimulating Factor 1 isoform in vivo prevents estrogen-deficiency bone loss in mice.Bone Res. 2017 Nov 14;5:17022. doi: 10.1038/boneres.2017.22. eCollection 2017. Bone Res. 2017. PMID: 29152381 Free PMC article.
-
Breast cancer-associated gene 3 interacts with Rac1 and augments NF-κB signaling in vitro, but has no effect on RANKL-induced bone resorption in vivo.Int J Mol Med. 2017 Oct;40(4):1067-1077. doi: 10.3892/ijmm.2017.3091. Epub 2017 Aug 4. Int J Mol Med. 2017. PMID: 28791343 Free PMC article.
References
-
- Massague J, Pandiella A. Membrane-anchored growth factors. Annu Rev Biochem. 1993;62:515–541. - PubMed
-
- Gommerman JL, Sittaro D, Klebasz NZ, Williams DA, Berger SA. Differential stimulation of c-Kit mutants by membrane-bound and soluble Steel Factor correlates with leukemic potential. Blood. 2000;96:3734–3742. - PubMed
-
- Caruana G, Ashman LK, Fujita J, Gonda TJ. Responses of the murine myeloid cell line FDC-P1 to soluble and membrane-bound forms of steel factor (SLF) Exp Hematol. 1993;21:761–768. - PubMed
-
- Friel J, Heberlein C, Itoh K, Ostertag W. Role of the stem cell factor (SCF) receptor and the alternative forms of its ligand (SCF) in the induction of long-term growth by stroma cells. Leukemia. 1997;11(Suppl 3):493–495. - PubMed
-
- Bazan JF. Genetic and structural homology of stem cell factor and macrophage colony-stimulating factor. Cell. 1991;65:9–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

