Crosstalk between adenosine A1 and β1-adrenergic receptors regulates translocation of PKCε in isolated rat cardiomyocytes
- PMID: 22105697
- PMCID: PMC3383333
- DOI: 10.1002/jcp.24008
Crosstalk between adenosine A1 and β1-adrenergic receptors regulates translocation of PKCε in isolated rat cardiomyocytes
Abstract
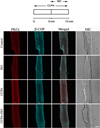
Adenosine A(1) receptor (A(1)R)-induced translocation of PKCε to transverse (t) tubular membranes in isolated rat cardiomyocytes is associated with a reduction in β(1)-adrenergic-stimulated contractile function. The PKCε-mediated activation of protein kinase D (PKD) by endothelin-1 is inhibited by β(1)-adrenergic stimulated protein kinase A (PKA) suggesting a similar mechanism of A(1)R signal transduction modulation by adrenergic agonists may exist in the heart. We have investigated the influence of β(1)-adrenergic stimulation on PKCε translocation elicited by A(1)R. Immunofluorescence imaging and Western blotting with PKCε and β-COP antibodies were used to quantify the co-localization of PKCε and t-tubular structures in isolated rat cardiomyocytes. The A(1)R agonist CCPA increased the co-localization of PKCε and t-tubules as detected by imaging. The β(1)-adrenergic receptor agonist isoproterenol (ISO) inhibited this effect of CCPA. Forskolin, a potent activator of PKA, mimicked, and H89, a pharmacological PKA inhibitor, and PKI, a membrane-permeable PKA peptide PKA inhibitor, attenuated the negative effect of ISO on the A(1)R-mediated PKCε translocation. Western blotting with isolated intact hearts revealed an increase in PKCε/β-COP co-localization induced by A(1)R. This increase was attenuated by the A(1)R antagonist DPCPX and ISO. The ISO-induced attenuation was reversed by H89. It is concluded that adrenergic stimulation inhibits A(1)R-induced PKCε translocation to the PKCε anchor site RACK2 constituent of a coatomer containing β-COP and associated with the t-tubular structures of the heart. In that this translocation has been previously associated with the antiadrenergic property of A(1)R, it is apparent that the interactive effects of adenosine and β(1)-adrenergic agonists on function are complex in the heart.
Copyright © 2011 Wiley Periodicals, Inc.
Figures





References
-
- Csukai M, Chen C-H, DeMatteis MA, Mochly-Rosen D. The coatomer protein β'COP: a selective binding protein (RACK) for epsilon protein kinase C. J Biol Chem. 1997;272:29200–29206. - PubMed
-
- Disatnik M-H, Buraggi G, Mochly-Rosen D. Localization of protein kinase C isozymes in cardiac myocytes. Exp Cell Res. 1994;210:287–297. - PubMed
-
- Dobson JG., Jr Reduction by adenosine of the isoproterenol-induced increase in cyclic adenosine 3',5'-monophosphate formation and glycogen phosphorylase activity in rat heart muscle. Circ Res. 1978;43:785–792. - PubMed
-
- Dobson JG., Jr Adenosine reduces catecholamine contractile responses in oxygenated and hypoxic atria. Am J Physiol. 1983a;245:H468–H474. - PubMed
-
- Dobson JG., Jr Mechanism of adenosine inhibition of catecholamine-induced elicited responses in heart. Circ Res. 1983b;52:151–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

