Terpene biosynthesis: modularity rules
- PMID: 22105807
- PMCID: PMC3769779
- DOI: 10.1002/anie.201103110
Terpene biosynthesis: modularity rules
Abstract
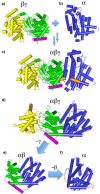
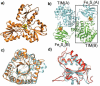
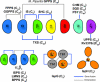
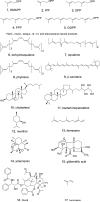
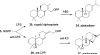
Terpenes are the largest class of small-molecule natural products on earth, and the most abundant by mass. Here, we summarize recent developments in elucidating the structure and function of the proteins involved in their biosynthesis. There are six main building blocks or modules (α, β, γ, δ, ε, and ζ) that make up the structures of these enzymes: the αα and αδ head-to-tail trans-prenyl transferases that produce trans-isoprenoid diphosphates from C(5) precursors; the ε head-to-head prenyl transferases that convert these diphosphates into the tri- and tetraterpene precursors of sterols, hopanoids, and carotenoids; the βγ di- and triterpene synthases; the ζ head-to-tail cis-prenyl transferases that produce the cis-isoprenoid diphosphates involved in bacterial cell wall biosynthesis; and finally the α, αβ, and αβγ terpene synthases that produce plant terpenes, with many of these modular enzymes having originated from ancestral α and β domain proteins. We also review progress in determining the structure and function of the two 4Fe-4S reductases involved in formation of the C(5) diphosphates in many bacteria, where again, highly modular structures are found.
Copyright © 2012 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures






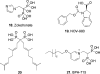
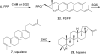

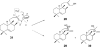
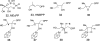
Similar articles
-
Isoprenyl diphosphate synthases: the chain length determining step in terpene biosynthesis.Planta. 2019 Jan;249(1):9-20. doi: 10.1007/s00425-018-3052-1. Epub 2018 Nov 22. Planta. 2019. PMID: 30467632 Review.
-
Assembly-Line Catalysis in Bifunctional Terpene Synthases.Acc Chem Res. 2021 Oct 19;54(20):3780-3791. doi: 10.1021/acs.accounts.1c00296. Epub 2021 Jul 13. Acc Chem Res. 2021. PMID: 34254507 Free PMC article. Review.
-
Targeting isoprenoid biosynthesis for drug discovery: bench to bedside.Acc Chem Res. 2010 Sep 21;43(9):1216-26. doi: 10.1021/ar100026v. Acc Chem Res. 2010. PMID: 20560544 Free PMC article.
-
Structural and Mechanistic Insight into Terpene Synthases that Catalyze the Irregular Non-Head-to-Tail Coupling of Prenyl Substrates.Chembiochem. 2019 Jan 2;20(1):29-33. doi: 10.1002/cbic.201800510. Epub 2018 Nov 22. Chembiochem. 2019. PMID: 30277292 Review.
-
Identification of Chimeric αβγ Diterpene Synthases Possessing both Type II Terpene Cyclase and Prenyltransferase Activities.Chembiochem. 2017 Nov 2;18(21):2104-2109. doi: 10.1002/cbic.201700445. Epub 2017 Sep 21. Chembiochem. 2017. PMID: 28869716
Cited by
-
Evolution of isoprenyl diphosphate synthase-like terpene synthases in fungi.Sci Rep. 2020 Sep 10;10(1):14944. doi: 10.1038/s41598-020-71219-z. Sci Rep. 2020. PMID: 32913319 Free PMC article.
-
Metal ions control product specificity of isoprenyl diphosphate synthases in the insect terpenoid pathway.Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):4194-9. doi: 10.1073/pnas.1221489110. Epub 2013 Feb 25. Proc Natl Acad Sci U S A. 2013. PMID: 23440195 Free PMC article.
-
Isoprenyl diphosphate synthases: the chain length determining step in terpene biosynthesis.Planta. 2019 Jan;249(1):9-20. doi: 10.1007/s00425-018-3052-1. Epub 2018 Nov 22. Planta. 2019. PMID: 30467632 Review.
-
Effects of Light and Temperature on the Metabolic Profiling of Two Habitat-Dependent Bloom-Forming Cyanobacteria.Metabolites. 2022 Apr 29;12(5):406. doi: 10.3390/metabo12050406. Metabolites. 2022. PMID: 35629910 Free PMC article.
-
Pinene-Based Oxidative Synthetic Toolbox for Scalable Polyester Synthesis.JACS Au. 2021 Oct 8;1(11):1949-1960. doi: 10.1021/jacsau.1c00312. eCollection 2021 Nov 22. JACS Au. 2021. PMID: 34849510 Free PMC article.
References
-
- Chappell J. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995;46:521–547.
-
- Poulter CD. Acc Chem Res. 1990;23:70–77.
-
- Berg JM, Stryer L. Biochemistry. 5th edition W H Freeman; New York: 2002.
-
- Bohlmann J, Keeling CI. Plant J. 2008;54:656–669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

