Comprehensive analysis of titin protein isoform and alternative splicing in normal and mutant rats
- PMID: 22105831
- PMCID: PMC6696936
- DOI: 10.1002/jcb.23459
Comprehensive analysis of titin protein isoform and alternative splicing in normal and mutant rats
Abstract
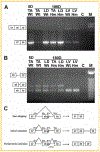
Titin is a giant protein with multiple functions in cardiac and skeletal muscles. Rat cardiac titin undergoes developmental isoform transition from the neonatal 3.7 MDa N2BA isoform to primarily the adult 2.97 MDa N2B isoform. An autosomal dominant mutation dramatically altered this transformation. Titins from eight skeletal muscles: Tibialis Anterior (TA), Longissimus Dorsi (LD) and Gastrocnemius (GA), Extensor Digitorum Longus (ED), Soleus (SO), Psoas (PS), Extensor Oblique (EO), and Diaphram (DI) were characterized in wild type and in homozygous mutant (Hm) rats with a titin splicing defect. Results showed that the developmental reduction in titin size is eliminated in the mutant rat so that the titins in all investigated skeletal muscles remain large in the adult. The alternative splicing of titin mRNA was found repressed by this mutation, a result consistent with the large titin isoform in the mutant. The developmental pattern of titin mRNA alternative splicing differs between heart and skeletal muscles. The retention of intron 49 reveals a possible mechanism for the absence of the N2B unique region in the expressed titin protein of skeletal muscle.
© 2011 Wiley Periodicals, Inc.
Figures

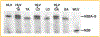





References
-
- Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S. 2001. The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89:1065–1072. - PubMed
-
- Berget SM. 1995. Exon recognition in vertebrate splicing. J Biol Chem 270: 2411–2414. - PubMed
-
- Centner T, Yano J, Kimura E, McElhinny AS, Pelin K, Witt CC, Bang ML, Trombitas K, Granzier H, Gregorio CC, Sorimachi H, Labeit S. 2001. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol 306:717–726. - PubMed
-
- Freiburg A, Trombitas K, Hell W, Cazorla O, Fougerousse F, Centner T, Kolmerer B, Witt C, Beckmann JS, Gregorio CC, Granzier H, Labeit S. 2000. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res 86:1114–1121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

