Signaling regulation of fetoplacental angiogenesis
- PMID: 22106098
- PMCID: PMC3288248
- DOI: 10.1530/JOE-11-0296
Signaling regulation of fetoplacental angiogenesis
Abstract
During normal pregnancy, dramatically increased placental blood flow is critical for fetal growth and survival as well as neonatal birth weights and survivability. This increased blood flow results from angiogenesis, vasodilatation, and vascular remodeling. Locally produced growth factors including fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor A (VEGFA) are key regulators of placental endothelial functions including cell proliferation, migration, and vasodilatation. However, the precise signaling mechanisms underlying such regulation in fetoplacental endothelium are less well defined, specifically with regard to the interactions amongst protein kinases (PKs), protein phosphatase, and nitric oxide (NO). Recently, we and other researchers have obtained solid evidence showing that different signaling mechanisms participate in FGF2- and VEGFA-regulated fetoplacental endothelial cell proliferation and migration as well as NO production. This review will briefly summarize currently available data on signaling mediating fetoplacental angiogenesis with a specific emphasis on PKs, ERK1/2, AKT1, and p38 MAPK and protein phosphatases, PPP2 and PPP3.
Conflict of interest statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures

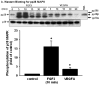


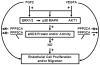
References
-
- Alessi DR, Gomez N, Moorhead G, Lewis T, Keyse SM, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Current Biology. 1995;5:283–295. - PubMed
-
- Alexander G. Birth weight of lambs. Influences and consequences. In: Elliott K, Knight J, editors. Ciba Foundation Symposium 27: Size at Birth. New York: Elsevier Publisher; 1974.
-
- Babaei S, Teichert-Kuliszewska K, Monge JC, Mohamed F, Bendeck MP, Stewart DJ. Role of nitric oxide in the angiogenic response in vitro to basic fibroblast growth factor. Circulation Research. 1998;82:1007–1015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

