Redefining the classification of AMPA-selective ionotropic glutamate receptors
- PMID: 22106175
- PMCID: PMC3300045
- DOI: 10.1113/jphysiol.2011.221689
Redefining the classification of AMPA-selective ionotropic glutamate receptors
Abstract
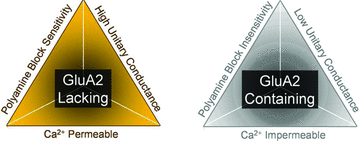
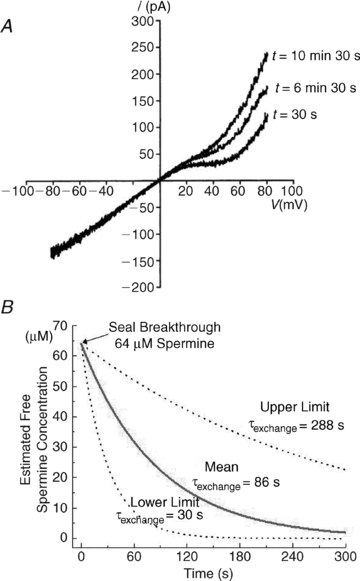
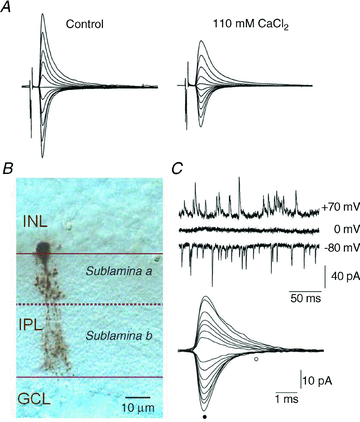
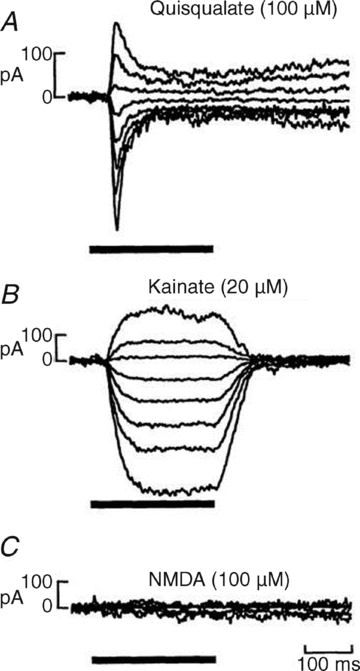
AMPA-type ionotropic glutamate receptors (iGluRs) represent the major excitatory neurotransmitter receptor in the developing and adult vertebrate CNS. They are crucial for the normal hardwiring of glutamatergic circuits but also fine tune synaptic strength by cycling into and out of synapses during periods of sustained patterned activity or altered homeostasis. AMPARs are grouped into two functionally distinct tetrameric assemblies based on the inclusion or exclusion of the GluA2 receptor subunit. GluA2-containing receptors are thought to be the most abundant AMPAR in the CNS, typified by their small unitary events, Ca(2+) impermeability and insensitivity to polyamine block. In contrast, GluA2-lacking AMPARs exhibit large unitary conductance, marked divalent permeability and nano- to micromolar polyamine affinity. Here, I review evidence for the existence of a third class of AMPAR which, though similarly Ca(2+) permeable, is characterized by its near-insensitivity to internal and external channel block by polyamines. This novel class of AMPAR is most notably found at multivesicular release synapses found in the avian auditory brainstem and mammalian retina. Curiously, these synapses lack NMDA-type iGluRs, which are conventionally associated with controlling AMPAR insertion. The lack of NMDARs suggests that a different set of rules may govern AMPAR cycling at these synapses. AMPARs with similar functional profiles are also found on some glial cells suggesting they may have a more widespread distribution in the mammalian CNS. I conclude by noting that modest changes to the ion-permeation pathway might be sufficient to retain divalent permeability whilst eliminating polyamine sensitivity. Consequently, this emerging AMPAR subclass need not be assembled from novel subunits, yet to be cloned, but could simply occur by varying the stoichiometry of existing proteins.
Figures
References
-
- Aizenman CD, Munoz-Elias G, Cline HT. Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron. 2002;34:623–634. - PubMed
-
- Almasieh A, Wilson A, Morquette B, Vargas J, Di PA. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2011 (in Press) - PubMed
-
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von ZM, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFa. Science. 2002;295:2282–2285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

