Modes of glutamate receptor gating
- PMID: 22106181
- PMCID: PMC3300047
- DOI: 10.1113/jphysiol.2011.223750
Modes of glutamate receptor gating
Abstract
The time course of excitatory synaptic currents, the major means of fast communication between neurons of the central nervous system, is encoded in the dynamic behaviour of post-synaptic glutamate-activated channels. First-pass attempts to explain the glutamate-elicited currents with mathematical models produced reaction mechanisms that included only the most basic functionally defined states: resting vs. liganded, closed vs. open, responsive vs. desensitized. In contrast, single-molecule observations afforded by the patch-clamp technique revealed an unanticipated kinetic multiplicity of transitions: from microseconds-lasting flickers to minutes-long modes. How these kinetically defined events impact the shape of the synaptic response, how they relate to rearrangements in receptor structure, and whether and how they are physiologically controlled represent currently active research directions. Modal gating, which refers to the slowest, least frequently observed ion-channel transitions, has been demonstrated for representatives of all ion channel families. However, reaction schemes have been largely confined to the short- and medium-range time scales. For glutamate receptors as well, modal gating has only recently come under rigorous scrutiny. This article reviews the evidence for modal gating of glutamate receptors and the still developing hypotheses about the mechanism(s) by which modal shifts occur and the ways in which they may impact the time course of synaptic transmission.
Figures
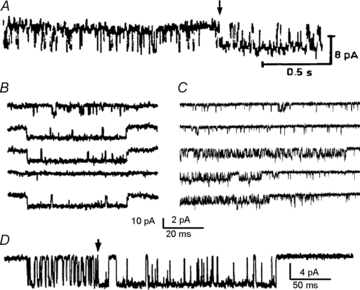
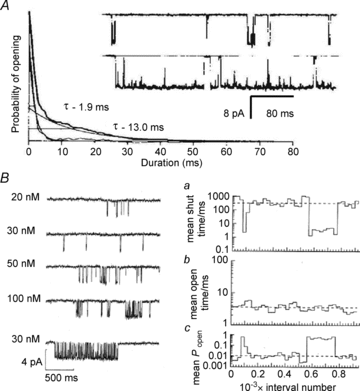



References
-
- Albright TD, Jessell TM, Kandel ER, Posner MI. Neural science: a century of progress and the mysteries that remain. Neuron. 2000;25:S1–S55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

