Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3
- PMID: 22106287
- PMCID: PMC3241768
- DOI: 10.1073/pnas.1103922108
Dual role of methionyl-tRNA synthetase in the regulation of translation and tumor suppressor activity of aminoacyl-tRNA synthetase-interacting multifunctional protein-3
Abstract
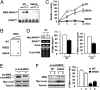
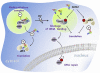
Mammalian methionyl-tRNA synthetase (MRS) plays an essential role in initiating translation by transferring Met to initiator tRNA (tRNA(i)(Met)). MRS also provides a cytosolic anchoring site for aminoacyl-tRNA synthetase-interacting multifunctional protein-3 (AIMP3)/p18, a potent tumor suppressor that is translocated to the nucleus for DNA repair upon DNA damage. However, the mechanism by which this enzyme mediates these two seemingly unrelated functions is unknown. Here we demonstrate that AIMP3 is released from MRS by UV irradiation-induced stress. Dissociation was induced by phosphorylation of MRS at Ser662 by general control nonrepressed-2 (GCN2) following UV irradiation. Substitution of Ser662 to Asp (S662D) induced a conformational change in MRS and significantly reduced its interaction with AIMP3. This mutant possessed significantly reduced MRS catalytic activity because of loss of tRNA(Met) binding, resulting in down-regulation of global translation. According to the Met incorporation assay using stable HeLa cells expressing MRS S662A or eukaryotic initiation factor-2 subunit-α (eIF2α) S51A, inactivation of GCN2-induced phosphorylation at eIF2α or MRS augmented the role of the other, suggesting a cross-talk between MRS and eIF2α for efficient translational inhibition. This work reveals a unique mode of regulation of global translation as mediated by aminoacyl-tRNA synthetase, specifically MRS, which we herein identified as a previously unidentified GCN2 substrate. In addition, our research suggests a dual role for MRS: (i) as a coregulator with eIF2α for GCN2-mediated translational inhibition; and (ii) as a coupler of translational inhibition and DNA repair following DNA damage by releasing bound tumor suppressor AIMP3 for its nuclear translocation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Lee SW, Cho BH, Park SG, Kim S. Aminoacyl-tRNA synthetase complexes: Beyond translation. J Cell Sci. 2004;117:3725–3734. - PubMed
-
- Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci. 2005;30:569–574. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

