Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties
- PMID: 22106347
- PMCID: PMC3257022
- DOI: 10.1182/blood-2011-08-370734
Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties
Abstract
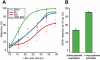
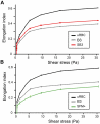
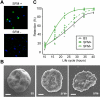
Infection of erythrocytes with the human malaria parasite, Plasmodium falciparum, results in dramatic changes to the host cell structure and morphology. The predicted functional localization of the STEVOR proteins at the erythrocyte surface suggests that they may be involved in parasite-induced modifications of the erythrocyte membrane during parasite development. To address the biologic function of STEVOR proteins, we subjected a panel of stevor transgenic parasites and wild-type clonal lines exhibiting different expression levels for stevor genes to functional assays exploring parasite-induced modifications of the erythrocyte membrane. Using this approach, we show that stevor expression impacts deformability of the erythrocyte membrane. This process may facilitate parasite sequestration in deep tissue vasculature.
Figures


References
-
- Maier AG, Cooke BM, Cowman AF, Tilley L. Malaria parasite proteins that remodel the host erythrocyte. Nat Rev Microbiol. 2009;7(5):341–354. - PubMed
-
- Nash GB, O'Brien E, Gordon-Smith EC, Dormandy JA. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood. 1989;74(2):855–861. - PubMed
-
- Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood. 2002;99(3):1060–1063. - PubMed
-
- Lavazec C, Sanyal S, Templeton TJ. Expression switching in the stevor and Pfmc-2TM superfamilies in Plasmodium falciparum. Mol Microbiol. 2007;64(6):1621–1634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

