Dynamic modulation of the kv2.1 channel by SRC-dependent tyrosine phosphorylation
- PMID: 22106938
- PMCID: PMC3272096
- DOI: 10.1021/pr200770v
Dynamic modulation of the kv2.1 channel by SRC-dependent tyrosine phosphorylation
Abstract
The voltage-gated K(+) channel Kv2.1 is expressed as a highly phosphorylated protein in most central neurons, where it plays a key role in regulating neuronal membrane excitability. Previous studies have shown that Kv2.1 channel activity is upregulated by Src-mediated phosphorylation through an unknown mechanism. However, a systematic analysis of the molecular mechanism of Kv2.1 channel phosphorylation by Src is lacking. Here, we show that tyrosine phosphorylation by Src plays a fundamental role in regulating Kv2.1-mediated K(+) current enhancement. We found that the level of expression of the Kv2.1 protein is increased by Src kinase. Using mass spectrometric proteomic techniques, we identified two novel phosphotyrosine sites, Y686 and Y810, in the cytoplasmic domains of Kv2.1. We found that Src-dependent phosphorylation at these sites affects Kv2.1 through distinct regulatory mechanisms. Whereas phosphorylation at Y686 regulates Kv2.1 activity similarly to the known site Y124, phosphorylation at Y810 plays a significant role in regulating the intracellular trafficking of Kv2.1 channels. Our results show that these two novel tyrosine phosphorylation sites of Kv2.1 are crucial to regulating diverse aspects of Kv2.1 channel function and provide novel insights into molecular mechanisms for the regulation of Src-dependent modulation of Kv2.1 channels.
Figures





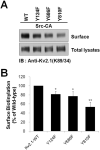
References
-
- Mohapatra DP, Park KS, Trimmer JS. Dynamic regulation of the voltage-gated Kv2.1 potassium channel by multisite phosphorylation. Biochem Soc Trans. 2007;35(Pt 5):1064–8. - PubMed
-
- Misonou H, Mohapatra D, Park E, Leung V, Zhen D, Misonou K, Anderson A, Trimmer J. Regulation of ion channel localization and phosphorylation by neuronal activity. Nature neuroscience. 2004;7(7):711–718. - PubMed
-
- Park KS, Mohapatra DP, Misonou H, Trimmer JS. Graded regulation of the Kv2.1 potassium channel by variable phosphorylation. Science. 2006;313(5789):976–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

