The transcription factor DLX3 regulates the osteogenic differentiation of human dental follicle precursor cells
- PMID: 22107079
- PMCID: PMC3396153
- DOI: 10.1089/scd.2011.0422
The transcription factor DLX3 regulates the osteogenic differentiation of human dental follicle precursor cells
Abstract
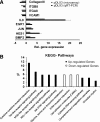
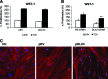
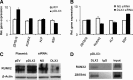
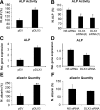
The transcription factor DLX3 plays a decisive role in bone development of vertebrates. In neural-crest derived stem cells from the dental follicle (DFCs), DLX3 is differentially expressed during osteogenic differentiation, while other osteogenic transcription factors such as DLX5 or RUNX2 are not highly induced. DLX3 has therefore a decisive role in the differentiation of DFCs, but its actual biological effects and regulation are unknown. This study investigated the DLX3-regulated processes in DFCs. After DLX3 overexpression, DFCs acquired a spindle-like cell shape with reorganized actin filaments. Here, marker genes for cell morphology, proliferation, apoptosis, and osteogenic differentiation were significantly regulated as shown in a microarray analysis. Further experiments showed that DFCs viability is directly influenced by the expression of DLX3, for example, the amount of apoptotic cells was increased after DLX3 silencing. This transcription factor stimulates the osteogenic differentiation of DFCs and regulates the BMP/SMAD1-pathway. Interestingly, BMP2 did highly induce DLX3 and reverse the inhibitory effect of DLX3 silencing in osteogenic differentiation. However, after DLX3 overexpression in DFCs, a BMP2 supplementation did not improve the expression of DLX3 and the osteogenic differentiation. In conclusion, DLX3 influences cell viability and regulates osteogenic differentiation of DFCs via a BMP2-dependent pathway and a feedback control.
Figures



Similar articles
-
A protein kinase A (PKA)/β-catenin pathway sustains the BMP2/DLX3-induced osteogenic differentiation in dental follicle cells (DFCs).Cell Signal. 2015 Mar;27(3):598-605. doi: 10.1016/j.cellsig.2014.12.008. Epub 2014 Dec 19. Cell Signal. 2015. PMID: 25530217
-
NOTCH1 signaling regulates the BMP2/DLX-3 directed osteogenic differentiation of dental follicle cells.Biochem Biophys Res Commun. 2014 Jan 10;443(2):500-4. doi: 10.1016/j.bbrc.2013.11.120. Epub 2013 Dec 7. Biochem Biophys Res Commun. 2014. PMID: 24321094
-
EGR1 supports the osteogenic differentiation of dental stem cells.Int Endod J. 2015 Feb;48(2):185-92. doi: 10.1111/iej.12299. Epub 2014 Jun 25. Int Endod J. 2015. PMID: 24749562
-
Mechanisms during Osteogenic Differentiation in Human Dental Follicle Cells.Int J Mol Sci. 2022 May 25;23(11):5945. doi: 10.3390/ijms23115945. Int J Mol Sci. 2022. PMID: 35682637 Free PMC article. Review.
-
Evaluation of Current Studies to Elucidate Processes in Dental Follicle Cells Driving Osteogenic Differentiation.Biomedicines. 2023 Oct 13;11(10):2787. doi: 10.3390/biomedicines11102787. Biomedicines. 2023. PMID: 37893160 Free PMC article. Review.
Cited by
-
Dental Follicle Cells: Roles in Development and Beyond.Stem Cells Int. 2019 Sep 15;2019:9159605. doi: 10.1155/2019/9159605. eCollection 2019. Stem Cells Int. 2019. PMID: 31636679 Free PMC article. Review.
-
Cytotoxicity and osteogenic potential of silicate calcium cements as potential protective materials for pulpal revascularization.Dent Mater. 2015 Dec;31(12):1510-22. doi: 10.1016/j.dental.2015.09.020. Epub 2015 Oct 19. Dent Mater. 2015. PMID: 26494267 Free PMC article.
-
Flightless-I governs cell fate by recruiting the SUMO isopeptidase SENP3 to distinct HOX genes.Epigenetics Chromatin. 2017 Mar 23;10:15. doi: 10.1186/s13072-017-0122-8. eCollection 2017. Epigenetics Chromatin. 2017. PMID: 28344658 Free PMC article.
-
Classical isoforms of protein kinase C (PKC) and Akt regulate the osteogenic differentiation of human dental follicle cells via both β-catenin and NF-κB.Stem Cell Res Ther. 2021 Apr 14;12(1):242. doi: 10.1186/s13287-021-02313-w. Stem Cell Res Ther. 2021. PMID: 33853677 Free PMC article.
-
Temporal Modulation of DNA Methylation and Gene Expression in Monolayer and 3D Spheroids of Dental Pulp Stem Cells during Osteogenic Differentiation: A Comparative Study.Tissue Eng Regen Med. 2022 Dec;19(6):1267-1282. doi: 10.1007/s13770-022-00485-x. Epub 2022 Oct 11. Tissue Eng Regen Med. 2022. PMID: 36221017 Free PMC article.
References
-
- Ten Cate AR. The development of the periodontium: a largely ectomesenchymally derived unit. Periodontol 2000. 1997;13:9–19. - PubMed
-
- Morsczeck C. Gotz W. Schierholz J. Zeilhofer F. Kuhn U. Mohl C. Sippel C. Hoffmann KH. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–165. - PubMed
-
- Völlner F. Ernst W. Driemel O. Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009;77:433–441. - PubMed
-
- Kemoun P. Laurencin-Dalicieux S. Rue J. Farges JC. Gennero I. Conte-Auriol F. Briand-Mesange F. Gadelorge M. Arzate H. Narayanan AS. Brunel G. Salles JP. Human dental follicle cells acquire cementoblast features under stimulation by BMP-2/-7 and enamel matrix derivatives (EMD) in vitro. Cell Tissue Res. 2007;329:283–294. - PubMed
-
- Thesleff I. Mikkola M. The role of growth factors in tooth development. Int Rev Cytol. 2002;217:93–135. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

