Phase I/II study of sagopilone (ZK-EPO) plus carboplatin in women with recurrent platinum-sensitive ovarian cancer
- PMID: 22108514
- PMCID: PMC3251849
- DOI: 10.1038/bjc.2011.499
Phase I/II study of sagopilone (ZK-EPO) plus carboplatin in women with recurrent platinum-sensitive ovarian cancer
Abstract
Background: Sagopilone is the first fully synthetic epothilone in clinical development and has demonstrated promising preclinical activity. This phase I/II, prospective, open-label trial investigated the efficacy and safety of sagopilone plus carboplatin in patients with recurrent platinum-sensitive ovarian cancer (OC).
Methods: In phase I (dose-escalation stage), patients with OC recurring at least 6 months after platinum-containing chemotherapy received 3-h infusions of sagopilone (initial dose of 12 mg m(-2)) followed by carboplatin every 3 weeks, for 2-6 treatment courses. Patients enrolled in phase II received 3-h infusions of 16 mg m(-2) sagopilone. Efficacy was assessed using modified Response Evaluation Criteria in Solid Tumors (modRECIST) and Gynecologic Cancer InterGroup CA125 criteria. The safety and tolerability of sagopilone were also evaluated.
Results: In all, 45 patients received sagopilone at 12 mg m(-2) or 16 mg m(-2). There were 29 confirmed tumour responses (21 modRECIST and 8 CA125) across both treatment groups, indicating that the primary objective of the study was reached. The main adverse events (AEs) reported were peripheral neuropathy (75.6%), fatigue (71.1%) and nausea (64.4%). Grade ≥3 AEs occurred in 35 patients (77.8%). No deaths related to the study drug were reported.
Conclusion: Sagopilone in combination with carboplatin was effective and toxicities were manageable in patients with recurrent platinum-sensitive OC.
Conflict of interest statement
R Patel, C Verschraegen, P Celano, J Burke, S Plaxe, P Ghatage and Y Wang declare no conflict of interest. M Giurescu (Senior Director), C Stredder (Global Study Manager) and T Schmelter (Study Statistician) are all employees of Bayer HealthCare Pharmaceuticals. S McMeekin received funding from Bayer HealthCare Pharmaceuticals for trial enrollment and has participated in advisory boards for Bayer HealthCare Pharmaceuticals.
Figures

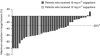

References
-
- Arnold D, Voigt W, Kiewe P, Behrmann C, Lindemann S, Reif S, Wiesinger H, Giurescu M, Thiel E, Schmoll H-J (2009) Weekly administration of sagopilone (ZK-EPO), a fully synthetic epothilone, in patients with refractory solid tumours: results of a phase I trial. Br J Cancer 101: 1241–1247 - PMC - PubMed
-
- Bafaloukos D, Linardou H, Aravantinos G, Papadimitriou C, Bamias A, Fountzilas G, Kalofonos HP, Kosmidis P, Timotheadou E, Makatsoris T, Samantas E, Briasoulis E, Christodoulou C, Papakostas P, Pectasides D, Dimopoulos AM (2010) A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Med 8: 3. - PMC - PubMed
-
- Beer T, Smith DC, Hussain A, Alonso M, Neerukonda L, Hauke R, Wang Y, Giurescu M (2008) Phase II study of first-line sagopilone combined with prednisone in patients with metastatic androgen-independent prostate cancer (AIPC). Ann Oncol 19(Suppl 8), Abstract 616P, pviii198
-
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ (2002) Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 20: 1248–1259 - PubMed
-
- Cantù MG, Buda A, Parma G, Rossi R, Floriani I, Bonazzi C, Dell'Anna T, Torri V, Colombo N (2002) Randomized controlled trial of single-agent paclitaxel versus cyclophosphamide, doxorubicin, and cisplatin in patients with recurrent ovarian cancer who responded to first-line platinum-based regimens. J Clin Oncol 20: 1232–1237 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

