Age-related accumulation of non-heme ferric and ferrous iron in mouse ovarian stroma visualized by sensitive non-heme iron histochemistry
- PMID: 22108647
- PMCID: PMC3351130
- DOI: 10.1369/0022155411431734
Age-related accumulation of non-heme ferric and ferrous iron in mouse ovarian stroma visualized by sensitive non-heme iron histochemistry
Abstract
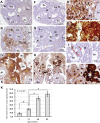
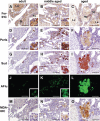
Sensitive non-heme iron histochemistry--namely, the perfusion-Perls method and perfusion-Turnbull method--was applied to study the distribution and age-related accumulation of non-heme ferric iron and ferrous iron in mouse ovary. Light and electron microscopic studies revealed that non-heme ferric iron is distributed predominantly in stromal tissue, especially in macrophages. By contrast, the distribution of non-heme ferrous iron was restricted to a few ovoid macrophages. Aged ovaries exhibited remarkable non-heme iron accumulation in all stromal cells. In particular, non-heme ferrous iron level was increased in stromal tissue, suggestive of increased levels of redox-active iron, which can promote oxidative stress. Moreover, intense localization of both non-heme ferric and ferrous iron was observed in aggregated large stromal cells that were then characterized as ceroid-laden enlarged macrophages with frothy cytoplasm. Intraperitoneal iron overload in adult mice resulted in non-heme iron deposition in the entire stroma and generation of enlarged macrophages, suggesting that excessive iron accumulation induced macrophage morphological changes. The data indicated that non-heme iron accumulation in ovarian stromal tissue may be related to aging of the ovary due to increasing oxidative stress.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures



References
-
- Agarwal A, Gupta S, Sikka S. 2006. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol. 18:325–332 - PubMed
-
- Aleshire SL, Osteen KG, Maxson WS, Entman SS, Bradley CA, Parl FF. 1989. Localization of transferrin and its receptor in ovarian follicular cells: morphologic studies in relation to follicular development. Fertil Steril. 51:444–449 - PubMed
-
- Asano Y, Meguro R, Odagiri S, Li C, Iwatsuki H, Shoumura K. 2006. Visualization of non-heme ferric and ferrous iron by highly sensitive non-heme iron histochemistry in the stress-induced acute gastric lesions in the rat. Histochem Cell Biol. 125:515–525 - PubMed
-
- Behrman HR, Kodaman PH, Preston SL, Gao S. 2001. Oxidative stress and the ovary. J Soc Gynecol Investig. 8(1 Suppl Proceedings):40–42 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

