Niche adaptation by expansion and reprogramming of general transcription factors
- PMID: 22108796
- PMCID: PMC3261711
- DOI: 10.1038/msb.2011.87
Niche adaptation by expansion and reprogramming of general transcription factors
Abstract
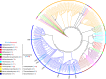
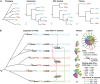
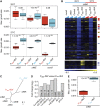
Numerous lineage-specific expansions of the transcription factor B (TFB) family in archaea suggests an important role for expanded TFBs in encoding environment-specific gene regulatory programs. Given the characteristics of hypersaline lakes, the unusually large numbers of TFBs in halophilic archaea further suggests that they might be especially important in rapid adaptation to the challenges of a dynamically changing environment. Motivated by these observations, we have investigated the implications of TFB expansions by correlating sequence variations, regulation, and physical interactions of all seven TFBs in Halobacterium salinarum NRC-1 to their fitness landscapes, functional hierarchies, and genetic interactions across 2488 experiments covering combinatorial variations in salt, pH, temperature, and Cu stress. This systems analysis has revealed an elegant scheme in which completely novel fitness landscapes are generated by gene conversion events that introduce subtle changes to the regulation or physical interactions of duplicated TFBs. Based on these insights, we have introduced a synthetically redesigned TFB and altered the regulation of existing TFBs to illustrate how archaea can rapidly generate novel phenotypes by simply reprogramming their TFB regulatory network.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G (2006) Engineering yeast transcription machinery for improved ethanol tolerance and production. Science 314: 1565–1568 - PubMed
-
- Bandyopadhyay S, Mehta M, Kuo D, Sung MK, Chuang R, Jaehnig EJ, Bodenmiller B, Licon K, Copeland W, Shales M, Fiedler D, Dutkowski J, Guenole A, van Attikum H, Shokat KM, Kolodner RD, Huh WK, Aebersold R, Keogh MC, Krogan NJ et al. (2010) Rewiring of genetic networks in response to DNA damage. Science 330: 1385–1389 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

