Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy
- PMID: 22108823
- PMCID: PMC4526218
- DOI: 10.1158/0008-5472.CAN-11-1782
Potent induction of tumor immunity by combining tumor cryoablation with anti-CTLA-4 therapy
Abstract
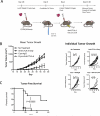
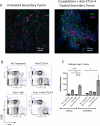
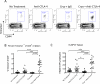
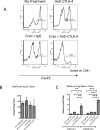
Thermal ablation to destroy tumor tissue may help activate tumor-specific T cells by elevating the presentation of tumor antigens to the immune system. However, the antitumor activity of these T cells may be restrained by their expression of the inhibitory T-cell coreceptor CTLA-4, the target of the recently U.S. Food and Drug Administration-approved antibody drug ipilumimab. By relieving this restraint, CTLA-4-blocking antibodies such as ipilumimab can promote tumor rejection, but the full scope of their most suitable applications has yet to be fully determined. In this study, we offer a preclinical proof-of-concept in the TRAMP C2 mouse model of prostate cancer that CTLA-4 blockade cooperates with cryoablation of a primary tumor to prevent the outgrowth of secondary tumors seeded by challenge at a distant site. Although growth of secondary tumors was unaffected by cryoablation alone, the combination treatment was sufficient to slow growth or trigger rejection. In addition, secondary tumors were highly infiltrated by CD4(+) T cells and CD8(+) T cells, and there was a significant increase in the ratio of intratumoral T effector cells to CD4(+)FoxP3(+) T regulatory cells, compared with monotherapy. These findings documented for the first time an effect of this immunotherapeutic intervention on the intratumoral accumulation and systemic expansion of CD8(+) T cells specific for the TRAMP C2-specific antigen SPAS-1. Although cryoablation is currently used to treat a targeted tumor nodule, our results suggest that combination therapy with CTLA-4 blockade will augment antitumor immunity and rejection of tumor metastases in this setting.
Figures


References
-
- Weld KJ, Landman J. Comparison of cryoablation, radiofrequency ablation and high-intensity focused ultrasound for treating small renal tumours. BJU Int. 2005;96:1224–9. - PubMed
-
- Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–86. - PubMed
-
- Link RE, Permpongkosol S, Gupta A, Jarrett TW, Solomon SB, Kavoussi LR. Cost analysis of open, laparoscopic, and percutaneous treatment options for nephron-sparing surgery. J Endourol. 2006;20:782–9. - PubMed
-
- Maybody M, Solomon SB. Image-guided percutaneous cryoablation of renal tumors. Tech Vasc Interv Radiol. 2007;10:140–8. - PubMed
-
- Bahn DK, Lee F, Badalament R, Kumar A, Greski J, Chernick M. Targeted cryoablation of the prostate: 7-year outcomes in the primary treatment of prostate cancer. Urology. 2002;60:3–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

