The golden age: gold nanoparticles for biomedicine
- PMID: 22109657
- PMCID: PMC5876014
- DOI: 10.1039/c1cs15237h
The golden age: gold nanoparticles for biomedicine
Abstract
Gold nanoparticles have been used in biomedical applications since their first colloidal syntheses more than three centuries ago. However, over the past two decades, their beautiful colors and unique electronic properties have also attracted tremendous attention due to their historical applications in art and ancient medicine and current applications in enhanced optoelectronics and photovoltaics. In spite of their modest alchemical beginnings, gold nanoparticles exhibit physical properties that are truly different from both small molecules and bulk materials, as well as from other nanoscale particles. Their unique combination of properties is just beginning to be fully realized in range of medical diagnostic and therapeutic applications. This critical review will provide insights into the design, synthesis, functionalization, and applications of these artificial molecules in biomedicine and discuss their tailored interactions with biological systems to achieve improved patient health. Further, we provide a survey of the rapidly expanding body of literature on this topic and argue that gold nanotechnology-enabled biomedicine is not simply an act of 'gilding the (nanomedicinal) lily', but that a new 'Golden Age' of biomedical nanotechnology is truly upon us. Moving forward, the most challenging nanoscience ahead of us will be to find new chemical and physical methods of functionalizing gold nanoparticles with compounds that can promote efficient binding, clearance, and biocompatibility and to assess their safety to other biological systems and their long-term term effects on human health and reproduction (472 references).
Figures


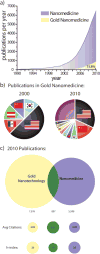



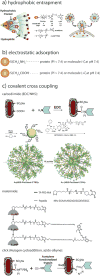
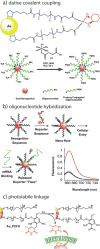
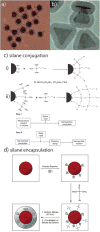







References
-
- Greenwood NN, Earnshaw A. Chemistry of the Elements. 2nd. Butterworth-Heinemann; Burlington, MA: 1997.
-
- Chen MS, Goodman DW. Science. 2004;306:252–255. - PubMed
-
- Valden M, Lai X, Goodman DW. Science. 1998;281:1647–1650. - PubMed
-
- Green IX, Tang W, Neurock M, Yates JT. Science. 2011;333:736–739. - PubMed
-
- Shaw CF. Chem Rev. 1999;99:2589–2600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

