Signaling by sensory receptors
- PMID: 22110046
- PMCID: PMC3249628
- DOI: 10.1101/cshperspect.a005991
Signaling by sensory receptors
Abstract
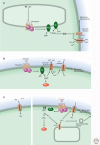
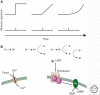
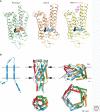
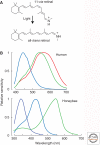
Sensory systems detect small molecules, mechanical perturbations, or radiation via the activation of receptor proteins and downstream signaling cascades in specialized sensory cells. In vertebrates, the two principal categories of sensory receptors are ion channels, which mediate mechanosensation, thermosensation, and acid and salt taste; and G-protein-coupled receptors (GPCRs), which mediate vision, olfaction, and sweet, bitter, and umami tastes. GPCR-based signaling in rods and cones illustrates the fundamental principles of rapid activation and inactivation, signal amplification, and gain control. Channel-based sensory systems illustrate the integration of diverse modulatory signals at the receptor, as seen in the thermosensory/pain system, and the rapid response kinetics that are possible with direct mechanical gating of a channel. Comparisons of sensory receptor gene sequences reveal numerous examples in which gene duplication and sequence divergence have created novel sensory specificities. This is the evolutionary basis for the observed diversity in temperature- and ligand-dependent gating among thermosensory channels, spectral tuning among visual pigments, and odorant binding among olfactory receptors. The coding of complex external stimuli by a limited number of sensory receptor types has led to the evolution of modality-specific and species-specific patterns of retention or loss of sensory information, a filtering operation that selectively emphasizes features in the stimulus that enhance survival in a particular ecological niche. The many specialized anatomic structures, such as the eye and ear, that house primary sensory neurons further enhance the detection of relevant stimuli.
Figures

References
-
- Abrahamson EW, Japar SM 1972. Principles of the interaction of light and matter. In Handbook of sensory physiology. VII/1: Photochemistry of vision (ed. Dartnall HJA), pp. 1–32 Springer-Verlag, Berlin
-
- Arshavsky VY, Lamb TD, Pugh EN 2002. G proteins and phototransduction. Annu Rev Physiol 64: 153–187 - PubMed
-
- Bakken GS, Krochmal AR 2007. The imaging properties and sensitivity of the facial pit of pitvipers as determined by optical and heat-transfer analysis. J Exp Biol 210: 2801–2810 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
