Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells
- PMID: 22110168
- PMCID: PMC3244040
- DOI: 10.1084/jem.20111490
Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells
Abstract
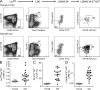
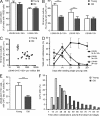
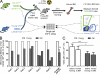
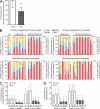
Hematopoietic stem cell (HSC) populations change with aging, but the extent to which this is caused by qualitative versus quantitative alterations in HSC subtypes is unclear. Using clonal assays, in this study we show that the aging HSC compartment undergoes both quantitative and qualitative changes. We observed a variable increase of HSC pool size with age, accompanied by the accumulation of predominantly myeloid-biased HSCs that regenerate substantially fewer mature progeny than young myeloid-biased HSCs and exhibit reduced self-renewal activity as measured by long-term secondary transplantation. Old HSCs had a twofold reduction in marrow-homing efficiency and a similar decrease in functional frequency as measured using long-term transplantation assays. Similarly, old HSCs had a twofold reduced seeding efficiency and a significantly delayed proliferative response compared with young HSCs in long-term stromal cell co-cultures but were indistinguishable in suspension cultures. We show that these functional defects are characteristics of most or all old HSCs and are not indicative of a nonfunctional subset of cells that express HSC markers. Furthermore, we demonstrate that cells with functional properties of old HSCs can be generated directly from young HSCs by extended serial transplantation, which is consistent with the possibility that they arise through a process of cellular aging.
Figures

References
-
- Beerman I., Bhattacharya D., Zandi S., Sigvardsson M., Weissman I.L., Bryder D., Rossi D.J. 2010a. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc. Natl. Acad. Sci. USA. 107:5465–5470 10.1073/pnas.1000834107 - DOI - PMC - PubMed
-
- Breems D.A., Blokland E.A., Neben S., Ploemacher R.E. 1994. Frequency analysis of human primitive haematopoietic stem cell subsets using a cobblestone area forming cell assay. Leukemia. 8:1095–1104 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

