Rho kinase proteins--pleiotropic modulators of cell survival and apoptosis
- PMID: 22110183
- PMCID: PMC3226732
Rho kinase proteins--pleiotropic modulators of cell survival and apoptosis
Abstract
Rho kinase (ROCK) proteins are Rho-GTPase activated serine/threonine kinases that function as modulators of actin-myosin cytoskeletal dynamics via regulation of Lin11, Isl-1 & Mec-3 domain (LIM) kinase, myosin light chain (MLC), and MLC phosphatase. A strong correlation between cytoskeletal rearrangements and tumor cell invasion, metastasis, and deregulated microenvironment interaction has been reported in the literature, and the utilization of pharmacological inhibitors of ROCK signaling for the treatment of cancer is actively being pursued by a number of pharmaceutical companies. Indeed, in many preclinical models ROCK inhibitors have shown remarkable efficacy in reducing tumor growth and metastasis. Interestingly, ROCK signaling has been shown to be either pro-apoptotic or pro-survival in a cell type and context dependent manner, though the molecular mechanisms controlling ROCK-mediated cell fate decisions are unknown. This review summarizes the many pleiotropic roles of ROCK signaling in survival and apoptosis, and suggests that controlled modulation of ROCK activity in tumor cells has the potential to significantly affect tumor survival and patient outcome.
Figures
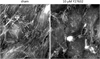
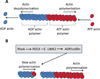
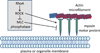
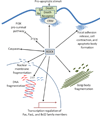
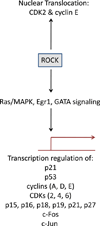
References
-
- Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
