Coordinate Regulation of Neurite Outgrowth by LRRK2 and Its Interactor, Rab5
- PMID: 22110348
- PMCID: PMC3214775
- DOI: 10.5607/en.2010.19.2.97
Coordinate Regulation of Neurite Outgrowth by LRRK2 and Its Interactor, Rab5
Abstract
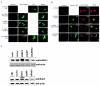
Neurite outgrowth and its maintenance are essential aspects of neuronal cells for their connectivity and communication with other neurons. Recent studies showed that over-expression of either leucine-rich repeat kinase 2 (LRRK2), whose mutations are associated with familial Parkinson's disease (PD), or Rab5b, an early endosomal marker protein, induces reduction in neurite length. Based on our recent findings that LRRK2 co-localizes and interacts with Rab5, we tested the hypothesis that LRRK2 and Rab5 may functionally interplay while controlling neurite outgrowth. Firstly, we confirmed previous reports that over-expression of either the LRRK2 PD-specific G2019S mutant or the Rab5 constitutively active Q79L mutant, but not of dominant negative N133I mutant, significantly reduces neurite outgrowth. Secondly, when over-expression of either LRRK2 wild type (WT) or G2019S was accompanied with over-expression of one of the Rab5 variants (WT, Q79L and N133I), or with down-regulation of Rab5, the reduction extent of its neurite length was similar to that of cells over-expressing LRRK2 alone, regardless of Rab5's status. Finally, we observed similar patterns of neurite length regulation in embryonic rat hippocampal neuron cultures. Taken together, our results suggest that LRRK2 and Rab5 functionally coordinate their regulation of neurite outgrowth and that LRRK2 is a more critical factor than Rab5.
Keywords: LRRK2; PC12 cells; Parkinson's disease; Rab5; neurite outgrowth.
Figures



References
-
- Daitoku H, Isida J, Fujiwara K, Nakajima T, Fukamizu A. Dimerization of small GTPase Rab5. Int J Mol Med. 2001;8:397–404. - PubMed
-
- de Hoop MJ, Huber LA, Stenmark H, Williamson E, Zerial M, Parton RG, Dotti CG. The involvement of the small GTP-binding protein Rab5a in neuronal endocytosis. Neuron. 1994;13:11–22. - PubMed

