Rapid Disruption of Cellular Integrity of Zinc-treated Astroglia Is Regulated by p38 MAPK and Ca-dependent Mechanisms
- PMID: 22110361
- PMCID: PMC3213738
- DOI: 10.5607/en.2011.20.1.45
Rapid Disruption of Cellular Integrity of Zinc-treated Astroglia Is Regulated by p38 MAPK and Ca-dependent Mechanisms
Abstract
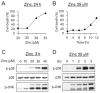
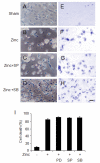
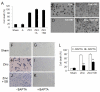
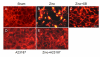
Cultured cortical primary astroglia treated with zinc died while rapidly detached from culture plates, a distinct part of zinc-treated astroglia. In the present study, we investigated the mechanism underlying the rapid change in the morphologic integrity of zinc-treated astroglia. Among the early cellular events occurring in zinc-treated astroglia, strong activation of p38 MAPK and JNK was evident. Although inhibitors of p38 (SB203580 and SB202190) or JNK (SP600125) did not protect zinc-insulted astroglia from cell death, the p38 inhibitors, but not the JNK inhibitor, suppressed actin filament and cell morphology disruption. The Ca(2+) ionophore, A23187, also suppressed actin filament and cell morphology disruption, but not cell death, of zinc-insulted astroglia. However, A23187 did not inhibit p38 MAPK activation in zinc-treated astroglia. Together these results suggest that zinc influx in astroglia results in rapid loss of the morphologic integrity via mechanisms regulated by p38 kinase and/or Ca(2+) signaling.
Keywords: actin filament; astroglia; morphology protection; p38 inhibitors; zinc.
Figures

References
-
- An WL, Bjorkdahl C, Liu R, Cowburn RF, Winblad B, Pei JJ. Mechanism of zinc-induced phosphorylation of p70 S6 kinase and glycogen synthase kinase 3beta in SH-SY5Y neuroblastoma cells. J Neurochem. 2005;92:1104–1115. - PubMed
-
- Arteaga S, Andrade-Cetto A, Cardenas R. Larrea tridentata (creosote bush), an abundant plant of Mexican and U.S. American deserts and its metabolite nordihydroguaiaretic acid. J Ethnopharmacol. 2005;98:231–239. - PubMed
-
- Carlier MF, Valentin-Ranc C, Combeau C, Fievez S, Pantoloni D. Actin polymerization: regulation by divalent metal ion and nucleotide binding, ATP hydrolysis and binding of myosin. Adv Exp Med Biol. 1994;358:71–81. - PubMed
-
- Carragher NO, Frame MC. Calpain: a role in cell transformation and migration. Int J Biochem Cell Biol. 2002;34:1539–1543. - PubMed
-
- Chae HJ, Ha HY, Im JY, Song JY, Park S, Han PL. JSAP1 is required for the cell adhesion and spreading of mouse embryonic fibroblasts. Biochem Biophys Res Commun. 2006;345:809–816. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

