Cholinergic control of visual categorization in macaques
- PMID: 22110428
- PMCID: PMC3215973
- DOI: 10.3389/fnbeh.2011.00073
Cholinergic control of visual categorization in macaques
Abstract
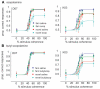
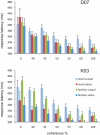
Acetylcholine (ACh) is a neurotransmitter acting via muscarinic and nicotinic receptors that is implicated in several cognitive functions and impairments, such as Alzheimer's disease. It is believed to especially affect the acquisition of new information, which is particularly important when behavior needs to be adapted to new situations and to novel sensory events. Categorization, the process of assigning stimuli to a category, is a cognitive function that also involves information acquisition. The role of ACh on categorization has not been previously studied. We have examined the effects of scopolamine, an antagonist of muscarinic ACh receptors, on visual categorization in macaque monkeys using familiar and novel stimuli. When the peripheral effects of scopolamine on the parasympathetic nervous system were controlled for, categorization performance was disrupted following systemic injections of scopolamine. This impairment was observed only when the stimuli that needed to be categorized had not been seen before. In other words, the monkeys were not impaired by the central action of scopolamine in categorizing a set of familiar stimuli (stimuli which they had categorized successfully in previous sessions). Categorization performance also deteriorated as the stimulus became less salient by an increase in the level of visual noise. However, scopolamine did not cause additional performance disruptions for difficult categorization judgments at lower coherence levels. Scopolamine, therefore, specifically affects the assignment of new exemplars to established cognitive categories, presumably by impairing the processing of novel information. Since we did not find an effect of scopolamine in the categorization of familiar stimuli, scopolamine had no significant central action on other cognitive functions such as perception, attention, memory, or executive control within the context of our categorization task.
Keywords: acetylcholine; categorization; cognition; cognitive; learning; macaque; muscarinic; scopolamine.
Figures




References
-
- Aigner T. G., Mitchell S. J., Aggleton J. P., DeLong M. R., Struble R. G., Price D. L., Wenk G. L., Mishkin M. (1987). Effects of scopolamine and physostigmine on recognition memory in monkeys with ibotenic-acid lesions of the nucleus basalis of Meynert. Psychopharmacology (Berl.) 9, 292–30010.1007/BF00210833 - DOI - PubMed
LinkOut - more resources
Full Text Sources

