Functional Anatomy of Non-REM Sleep
- PMID: 22110467
- PMCID: PMC3215999
- DOI: 10.3389/fneur.2011.00070
Functional Anatomy of Non-REM Sleep
Abstract
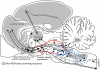
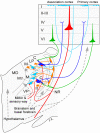
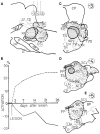
The state of non-REM sleep (NREM), or slow wave sleep, is associated with a synchronized EEG pattern in which sleep spindles and/or K complexes and high-voltage slow wave activity (SWA) can be recorded over the entire cortical surface. In humans, NREM is subdivided into stages 2 and 3-4 (presently named N3) depending on the proportions of each of these polygraphic events. NREM is necessary for normal physical and intellectual performance and behavior. An overview of the brain structures involved in NREM generation shows that the thalamus and the cerebral cortex are absolutely necessary for the most significant bioelectric and behavioral events of NREM to be expressed; other structures like the basal forebrain, anterior hypothalamus, cerebellum, caudal brain stem, spinal cord and peripheral nerves contribute to NREM regulation and modulation. In NREM stage 2, sustained hyperpolarized membrane potential levels resulting from interaction between thalamic reticular and projection neurons gives rise to spindle oscillations in the membrane potential; the initiation and termination of individual spindle sequences depends on corticothalamic activities. Cortical and thalamic mechanisms are also involved in the generation of EEG delta SWA that appears in deep stage 3-4 (N3) NREM; the cortex has classically been considered to be the structure that generates this activity, but delta oscillations can also be generated in thalamocortical neurons. NREM is probably necessary to normalize synapses to a sustainable basal condition that can ensure cellular homeostasis. Sleep homeostasis depends not only on the duration of prior wakefulness but also on its intensity, and sleep need increases when wakefulness is associated with learning. NREM seems to ensure cell homeostasis by reducing the number of synaptic connections to a basic level; based on simple energy demands, cerebral energy economizing during NREM sleep is one of the prevalent hypotheses to explain NREM homeostasis.
Keywords: NREM sleep homeostasis; caudal hypnogenic system; rostral hypnogenic system; sleep need; slow wave sleep; thalamus–cerebral cortex unit.
Figures





References
-
- Alam M. N., McGinty D., Szymusiak R. (1995). Neuronal discharge of preoptic/anterior hypothalamic thermosensitive neurons: relation to NREM sleep. Am. J. Physiol. 269, R1240–R1249 - PubMed
-
- Avendaño C., Stepniewska I., Rausell E., Reinoso-Suárez F. (1990). Segregation and heterogeneity of thalamic cell populations projecting to superficial layers of the posterior parietal cortex: a retrograde tracing study in the cat and monkey. Neuroscience 39, 547–559 10.1016/0306-4522(90)90242-V - DOI - PubMed
LinkOut - more resources
Full Text Sources
Medical

