Long circulating lectin conjugated paclitaxel loaded magnetic nanoparticles: a new theranostic avenue for leukemia therapy
- PMID: 22110595
- PMCID: PMC3217954
- DOI: 10.1371/journal.pone.0026803
Long circulating lectin conjugated paclitaxel loaded magnetic nanoparticles: a new theranostic avenue for leukemia therapy
Abstract
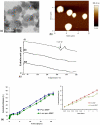
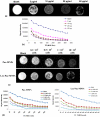
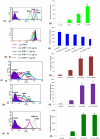
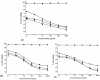
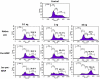
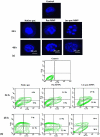
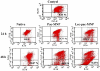
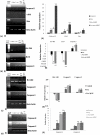
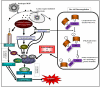
Amongst all leukemias, Bcr-Abl positive chronic myelogenous leukemia (CML) confers resistance to native drug due to multi drug resistance and also resistance to p53 and fas ligand pathways. In the present study, we have investigated the efficacy of microtubule stabilizing paclitaxel loaded magnetic nanoparticles (pac-MNPs) to ascertain its cytotoxic effect on Bcr-Abl positive K562 cells. For active targeted therapy, pac-MNPs were functionalized with lectin glycoprotein which resulted in higher cellular uptake and lower IC(50) value suggesting the efficacy of targeted delivery of paclitaxel. Both pac-MNPs and lectin conjugated pac-MNPs have a prolonged circulation time in serum suggesting increased bioavailability and therapeutics index of paclitaxel in vivo. Further, the molecular mechanism pertaining to pac-induced cytotoxicity was analyzed by studying the involvement of different apoptotic pathway proteins by immunoblotting and quantitative PCR. Our study revealed simultaneous activation of JNK pathway leading to Bcr-Abl instability and the extrinsic apoptotic pathway after pac-MNPs treatment in two Bcr-Abl positive cell lines. In addition, the MRI data suggested the potential application of MNPs as imaging agent. Thus our in vitro and in vivo results strongly suggested the pac-MNPs as a future prospective theranostic tool for leukemia therapy.
Conflict of interest statement
Figures
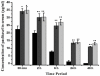

References
-
- Jacquel A, Herrant M, Legros L, Belhacene N, Luciano F, et al. Imatinib induces mitochondria-dependent apoptosis of the Bcr-Abl-positive K562 cell line and its differentiation toward the erythroid lineage. Faseb J. 2003;17:2160–2162. - PubMed
-
- Terme M, Borg C, Guilhot F, Masurier C, Flament C, et al. BCR/ABL promotes dendritic cell-mediated natural killer cell activation. Cancer Res. 2005;65:6409–6417. - PubMed
-
- Nagata Y, Todokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. - PubMed
-
- Jia L, Patwari Y, Kelsey SM, Newland AC. Trail-induced apoptosis in Type I leukemic cells is not enhanced by overexpression of bax. Biochem Biophys Res Commun. 2001;283:1037–1045. - PubMed
-
- Knijn A, Brisdelli F, Ferretti A, Iorio E, Marcheggiani D, et al. Metabolic alterations in K562 cells exposed to taxol and tyrphostin AG957: 1H NMR and biochemical studies. Cell Biol Int. 2005;29:890–897. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

