The nuclear pore complex mediates binding of the Mig1 repressor to target promoters
- PMID: 22110603
- PMCID: PMC3215702
- DOI: 10.1371/journal.pone.0027117
The nuclear pore complex mediates binding of the Mig1 repressor to target promoters
Abstract
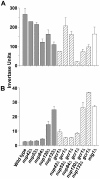
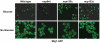
All eukaryotic cells alter their transcriptional program in response to the sugar glucose. In Saccharomyces cerevisiae, the best-studied downstream effector of this response is the glucose-regulated repressor Mig1. We show here that nuclear pore complexes also contribute to glucose-regulated gene expression. NPCs participate in glucose-responsive repression by physically interacting with Mig1 and mediating its function independently of nucleocytoplasmic transport. Surprisingly, despite its abundant presence in the nucleus of glucose-grown nup120Δ or nup133Δ cells, Mig1 has lost its ability to interact with target promoters. The glucose repression defect in the absence of these nuclear pore components therefore appears to result from the failure of Mig1 to access its consensus recognition sites in genomic DNA. We propose that the NPC contributes to both repression and activation at the level of transcription.
Conflict of interest statement
Figures






References
-
- Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–341. - PubMed
-
- Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am J Physiol. 1999;277:E1–10. - PubMed
-
- Hong YH, Varanasi US, Yang W, Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J Biol Chem. 2003;278:27495–27501. - PubMed
-
- Leff T. AMP-activated protein kinase regulates gene expression by direct phosphorylation of nuclear proteins. Biochem Soc Trans. 2003;31:224–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

