Depression screening and patient outcomes in cancer: a systematic review
- PMID: 22110613
- PMCID: PMC3215716
- DOI: 10.1371/journal.pone.0027181
Depression screening and patient outcomes in cancer: a systematic review
Abstract
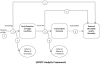
Background: Several practice guidelines recommend screening for depression in cancer care, but no systematic reviews have examined whether there is evidence that depression screening benefits cancer patients. The objective was to evaluate the potential benefits of depression screening in cancer patients by assessing the (1) accuracy of depression screening tools; (2) effectiveness of depression treatment; and (3) effect of depression screening, either alone or in the context of comprehensive depression care, on depression outcomes.
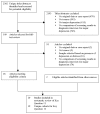
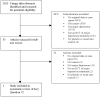
Methods: Data sources were CINAHL, Cochrane, EMBASE, ISI, MEDLINE, PsycINFO and SCOPUS databases through January 24, 2011; manual journal searches; reference lists; citation tracking; trial registry reviews. Articles on cancer patients were included if they (1) compared a depression screening instrument to a valid criterion for major depressive disorder (MDD); (2) compared depression treatment with placebo or usual care in a randomized controlled trial (RCT); (3) assessed the effect of screening on depression outcomes in a RCT.
Results: There were 19 studies of screening accuracy, 1 MDD treatment RCT, but no RCTs that investigated effects of screening on depression outcomes. Screening accuracy studies generally had small sample sizes (median = 17 depression cases) and used exploratory methods to set sample-specific cutoff scores that varied substantially across studies. A nurse-delivered intervention for MDD reduced depressive symptoms moderately (effect size = 0.37).
Conclusions: The one treatment study reviewed reported modest improvement in depressive symptoms, but no evidence was found on whether or not depression screening in cancer patients, either alone or in the context of optimal depression care, improves depression outcomes compared to usual care. Depression screening in cancer should be evaluated in a RCT in which all patients identified as depressed, either through screening or via physician recognition and referral in a control group, have access to comprehensive depression care.
Conflict of interest statement
Figures
References
-
- American Cancer Society. Cancer facts & figures 2010. Atlanta: American Cancer Society; 2010. 66
-
- Altekruse SF, Kosry CL, Krapcho M, Neyman N, Aminou R, et al. SEER cancer statistics review, 1975–2007. Bethesda, , MD: National Cancer Institute; 2011.
-
- National Comprehensive Cancer Network. Distress Management. 2008. NCCN clinical practice guidelines in oncology. http://www.nccn.org/professionals/physician_gls/PDF/distress.pdf.
-
- Institute of Medicine. Cancer care for the whole patient: meeting psychosocial health needs. Washington, DC: National Academy Press; 2007. 430
-
- Ng CG, Boks MP, Zainal NZ, De Wit NJ. The prevalence and pharmacotherapy of depression in cancer patients. J Affect Disord. 2011;131:1–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources