Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada
- PMID: 22110645
- PMCID: PMC3215749
- DOI: 10.1371/journal.pone.0027420
Estimation of the health impact and cost-effectiveness of influenza vaccination with enhanced effectiveness in Canada
Abstract
Introduction: The propensity for influenza viruses to mutate and recombine makes them both a familiar threat and a prototype emerging infectious disease. Emerging evidence suggests that the use of MF59-adjuvanted vaccines in older adults and young children enhances protection against influenza infection and reduces adverse influenza-attributable outcomes compared to unadjuvanted vaccines. The health and economic impact of such vaccines in the Canadian population are uncertain.
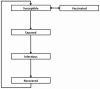
Methods: We constructed an age-structured compartmental model simulating the transmission of influenza in the Canadian population over a ten-year period. We compared projected health outcomes (quality-adjusted life years (QALY) lost), costs, and incremental cost-effectiveness ratios (ICERs) for three strategies: (i) current use of unadjuvanted trivalent influenza vaccine; (ii) use of MF59-adjuvanted influenza vaccine adults ≥65 in the Canadian population, and (iii) adjuvanted vaccine used in both older adults and children aged < 6.
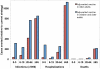
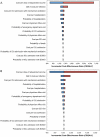
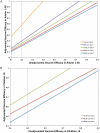
Results: In the base case analysis, use of adjuvanted vaccine in older adults was highly cost-effective (ICER = $2111/QALY gained), but such a program was "dominated" by a program that extended the use of adjuvanted vaccine to include young children (ICER = $1612/QALY). Results were similar whether or not a universal influenza immunization program was used in other age groups; projections were robust in the face of wide-ranging sensitivity analyses.
Interpretation: Based on the best available data, it is projected that replacement of traditional trivalent influenza vaccines with MF59-adjuvanted vaccines would confer substantial benefits to vaccinated and unvaccinated individuals, and would be economically attractive relative to other widely-used preventive interventions.
Conflict of interest statement
Figures
Similar articles
-
Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population.Hum Vaccin Immunother. 2018 Jun 3;14(6):1331-1341. doi: 10.1080/21645515.2018.1438792. Epub 2018 Feb 26. Hum Vaccin Immunother. 2018. PMID: 29425079 Free PMC article.
-
Cost-effectiveness analysis of quadrivalent versus trivalent influenza vaccine in Taiwan: A lifetime multi-cohort model.Hum Vaccin Immunother. 2017 Jan 2;13(1):81-89. doi: 10.1080/21645515.2016.1225636. Epub 2016 Sep 13. Hum Vaccin Immunother. 2017. PMID: 27624648 Free PMC article.
-
Cost effectiveness of vaccination against pandemic influenza in European countries: mathematical modelling analysis.BMJ. 2012 Jul 12;345:e4445. doi: 10.1136/bmj.e4445. BMJ. 2012. PMID: 22791791 Free PMC article.
-
Use of adjuvanted trivalent influenza vaccine in older-age adults: a systematic review of economic evidence.Hum Vaccin Immunother. 2019;15(5):1035-1047. doi: 10.1080/21645515.2019.1578597. Epub 2019 Mar 25. Hum Vaccin Immunother. 2019. PMID: 30735465 Free PMC article.
-
MF59 adjuvanted seasonal and pandemic influenza vaccines.Yakugaku Zasshi. 2011;131(12):1733-41. doi: 10.1248/yakushi.131.1733. Yakugaku Zasshi. 2011. PMID: 22129867 Review.
Cited by
-
Cost-effectiveness analysis of different seasonal influenza vaccines in the elderly Italian population.Hum Vaccin Immunother. 2018 Jun 3;14(6):1331-1341. doi: 10.1080/21645515.2018.1438792. Epub 2018 Feb 26. Hum Vaccin Immunother. 2018. PMID: 29425079 Free PMC article.
-
Integrating Transgenic Vector Manipulation with Clinical Interventions to Manage Vector-Borne Diseases.PLoS Comput Biol. 2016 Mar 10;12(3):e1004695. doi: 10.1371/journal.pcbi.1004695. eCollection 2016 Mar. PLoS Comput Biol. 2016. PMID: 26962871 Free PMC article.
-
Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion.Vaccines (Basel). 2023 Jun 11;11(6):1089. doi: 10.3390/vaccines11061089. Vaccines (Basel). 2023. PMID: 37376478 Free PMC article. Review.
-
Natural attack rate of influenza in unvaccinated children and adults: a meta-regression analysis.BMC Infect Dis. 2014 Dec 11;14:670. doi: 10.1186/s12879-014-0670-5. BMC Infect Dis. 2014. PMID: 25495228 Free PMC article.
-
Review of seasonal influenza in Canada: Burden of disease and the cost-effectiveness of quadrivalent inactivated influenza vaccines.Hum Vaccin Immunother. 2017 Apr 3;13(4):867-876. doi: 10.1080/21645515.2016.1251537. Epub 2016 Nov 18. Hum Vaccin Immunother. 2017. PMID: 27858509 Free PMC article. Review.
References
-
- Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. - PubMed
-
- Postma MJ, Jansema P, van Genugten ML, Heijnen ML, Jager JC, et al. Pharmacoeconomics of influenza vaccination for healthy working adults: reviewing the available evidence. Drugs. 2002;62:1013–1024. - PubMed
-
- O'Reilly FW, Stevens AB. Sickness absence due to influenza. Occup Med (Lond) 2002;52:265–269. - PubMed
-
- Keech M, Scott AJ, Ryan PJ. The impact of influenza and influenza-like illness on productivity and healthcare resource utilization in a working population. Occup Med (Lond) 1998;48:85–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

