Cell edges accumulate gamma tubulin complex components and nucleate microtubules following cytokinesis in Arabidopsis thaliana
- PMID: 22110647
- PMCID: PMC3212562
- DOI: 10.1371/journal.pone.0027423
Cell edges accumulate gamma tubulin complex components and nucleate microtubules following cytokinesis in Arabidopsis thaliana
Abstract
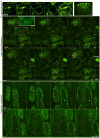
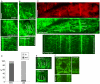
Microtubules emanate from distinct organizing centers in fungal and animal cells. In plant cells, by contrast, microtubules initiate from dispersed sites in the cell cortex, where they then self-organize into parallel arrays. Previous ultrastructural evidence suggested that cell edges participate in microtubule nucleation but so far there has been no direct evidence for this. Here we use live imaging to show that components of the gamma tubulin nucleation complex (GCP2 and GCP3) localize at distinct sites along the outer periclinal edge of newly formed crosswalls, and that microtubules grow predominantly away from these edges. These data confirm a role for cell edges in microtubule nucleation, and suggest that an asymmetric distribution of microtubule nucleation factors contributes to cortical microtubule organization in plants, in a manner more similar to other kingdoms than previously thought.
Conflict of interest statement
Figures

References
-
- Schmit AC. Acentrosomal microtubule nucleation in higher plants. Int Rev Cytol. 2002;220:257–289. - PubMed
-
- Wasteneys GO. Microtubule organization in the green kingdom: chaos or self-order? J Cell Sci. 2002;115:1345–1354. - PubMed
-
- Cleary AL, Hardham AR. Depolymerization of microtubule arrays in root tip cells by oryzalin and their recovery with modified nucleation patterns. Can J Bot. 1987;66:2353–2366.
-
- Wasteneys GO, Williamson RE. Reassembly of microtubules in Nitella tasmanica: assembly of cortical microtubules in branching clusters and its relevance to steady-state microtubule assembly. J Cell Sci. 1989;93:705–714.
-
- Falconer MM, Donaldson G, Seagull RW. MTOCs in higher plant cells: an immunofluorescent study of microtubule assembly sites following depolymerization by APM. Protoplasma. 1988;144:46–55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

