Autophagy in human embryonic stem cells
- PMID: 22110659
- PMCID: PMC3215747
- DOI: 10.1371/journal.pone.0027485
Autophagy in human embryonic stem cells
Abstract
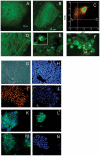
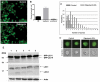
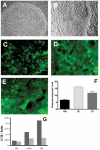
Autophagy (macroautophagy) is a degradative process that involves the sequestration of cytosolic material including organelles into double membrane vesicles termed autophagosomes for delivery to the lysosome. Autophagy is essential for preimplantation development of mouse embryos and cavitation of embryoid bodies. The precise roles of autophagy during early human embryonic development, remain however largely uncharacterized. Since human embryonic stem cells constitute a unique model system to study early human embryogenesis we investigated the occurrence of autophagy in human embryonic stem cells. We have, using lentiviral transduction, established multiple human embryonic stem cell lines that stably express GFP-LC3, a fluorescent marker for the autophagosome. Each cell line displays both a normal karyotype and pluripotency as indicated by the presence of cell types representative of the three germlayers in derived teratomas. GFP expression and labelling of autophagosomes is retained after differentiation. Baseline levels of autophagy detected in cultured undifferentiated hESC were increased or decreased in the presence of rapamycin and wortmannin, respectively. Interestingly, autophagy was upregulated in hESCs induced to undergo differentiation by treatment with type I TGF-beta receptor inhibitor SB431542 or removal of MEF secreted maintenance factors. In conclusion we have established hESCs capable of reporting macroautophagy and identify a novel link between autophagy and early differentiation events in hESC.
Conflict of interest statement
Figures

References
-
- Pera MF, Trounson AO. Human embryonic stem cells: prospects for development. Development. 2004;131:5515–5525. - PubMed
-
- Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, et al. A ubiquitin-like system mediates protein lipidation. Nature. 2000;408:488–492. - PubMed
-
- Cullinane M, Gong L, Li X, Lazar-Adler N, Tra T, et al. Stimulation of autophagy suppresses the intracellular survival of Burkholderia pseudomallei in mammalian cell lines. Autophagy. 2008;4:744–753. - PubMed
-
- Klionsky DJ, Cuervo AM, Seglen PO. Methods for monitoring autophagy from yeast to human. Autophagy. 2007;3:181–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

