A unique role of GATA1s in Down syndrome acute megakaryocytic leukemia biology and therapy
- PMID: 22110660
- PMCID: PMC3217966
- DOI: 10.1371/journal.pone.0027486
A unique role of GATA1s in Down syndrome acute megakaryocytic leukemia biology and therapy
Abstract
Background: Acute megakaryocytic leukemia (AMkL) in Down syndrome (DS) children is uniformly associated with somatic GATA1 mutations, which result in the synthesis of a shorter protein (GATA1s) with altered transactivation activity compared to the wild-type GATA1. It is not fully established whether leukemogenesis and therapeutic responses in DS AMkL patients are due to loss of the wild-type GATA1 or due to a unique function of GATA1s.
Methodology: Stable clones of CMK cells with decreased GATA1s or Bcl-2 levels were generated by using GATA1- or BCL-2-specific lentivirus shRNAs. In vitro ara-C, daunorubicin, and VP-16 cytotoxicities of the shRNA stable clones were determined by using the Cell Titer-blue reagent. Apoptosis and cell cycle distribution were determined by flow cytometry analysis. Changes in gene transcript levels were determined by gene expression microarray and/or real-time RT-PCR. Changes in protein levels were measured by Western blotting. In vivo binding of GATA1s to IL1A promoter was determined by chromatin immunoprecipitation assays.
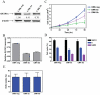
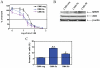
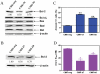
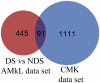
Results: Lentivirus shRNA knockdown of the GATA1 gene in the DS AMkL cell line, CMK (harbors a mutated GATA1 gene and only expresses GATA1s), resulting in lower GATA1s protein levels, promoted cell differentiation towards the megakaryocytic lineage and repressed cell proliferation. Increased basal apoptosis and sensitivities to ara-C, daunorubicin, and VP-16 accompanied by down-regulated Bcl-2 were also detected in the CMK GATA1 shRNA knockdown clones. Essentially the same results were obtained when Bcl-2 was knocked down with lentivirus shRNA in CMK cells. Besides Bcl-2, down-regulation of GATA1s also resulted in altered expression of genes (e.g., IL1A, PF4, and TUBB1) related to cell death, proliferation, and differentiation.
Conclusion: Our results suggest that GATA1s may facilitate leukemogenesis and potentially impact therapeutic responses in DS AMkL by promoting proliferation and survival, and by repressing megakaryocytic lineage differentiation, potentially by regulating expression of Bcl-2 protein and other relevant genes.
Conflict of interest statement
Figures


References
-
- Taub JW. Relationship of chromosome 21 and acute leukemia in children with Down syndrome. J Pediatr Hematol Oncol. 2001;23:175–178. - PubMed
-
- Ravindranath Y, Abella E, Krischer JP, Wiley J, Inoue S, et al. Acute myeloid leukemia (AML) in Down's syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992;80:2210–2214. - PubMed
-
- Lange BJ, Kobrinsky N, Barnard DR, Arthur DC, Buckley JD, et al. Distinctive demography, biology, and outcome of acute myeloid leukemia and myelodysplastic syndrome in children with Down syndrome: Children's Cancer Group Studies 2861 and 2891. Blood. 1998;91:608–615. - PubMed
-
- Gamis AS, Woods WG, Alonzo TA, Buxton A, Lange B, et al. Increased age at diagnosis has a significantly negative effect on outcome in children with Down syndrome and acute myeloid leukemia: a report from the Children's Cancer Group Study 2891. J Clin Oncol. 2003;21:3415–3422. - PubMed
-
- Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, et al. Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution's experience. Blood. 2001;97:3727–3732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

