Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway
- PMID: 22110680
- PMCID: PMC3217975
- DOI: 10.1371/journal.pone.0027586
Activation of GPR4 by acidosis increases endothelial cell adhesion through the cAMP/Epac pathway
Abstract
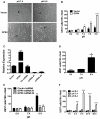
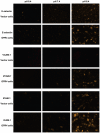
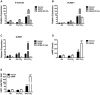
Endothelium-leukocyte interaction is critical for inflammatory responses. Whereas the tissue microenvironments are often acidic at inflammatory sites, the mechanisms by which cells respond to acidosis are not well understood. Using molecular, cellular and biochemical approaches, we demonstrate that activation of GPR4, a proton-sensing G protein-coupled receptor, by isocapnic acidosis increases the adhesiveness of human umbilical vein endothelial cells (HUVECs) that express GPR4 endogenously. Acidosis in combination with GPR4 overexpression further augments HUVEC adhesion with U937 monocytes. In contrast, overexpression of a G protein signaling-defective DRY motif mutant (R115A) of GPR4 does not elicit any increase of HUVEC adhesion, indicating the requirement of G protein signaling. Downregulation of GPR4 expression by RNA interference reduces the acidosis-induced HUVEC adhesion. To delineate downstream pathways, we show that inhibition of adenylate cyclase by inhibitors, 2',5'-dideoxyadenosine (DDA) or SQ 22536, attenuates acidosis/GPR4-induced HUVEC adhesion. Consistently, treatment with a cAMP analog or a G(i) signaling inhibitor increases HUVEC adhesiveness, suggesting a role of the G(s)/cAMP signaling in this process. We further show that the cAMP downstream effector Epac is important for acidosis/GPR4-induced cell adhesion. Moreover, activation of GPR4 by acidosis increases the expression of vascular adhesion molecules E-selectin, VCAM-1 and ICAM-1, which are functionally involved in acidosis/GPR4-mediated HUVEC adhesion. Similarly, hypercapnic acidosis can also activate GPR4 to stimulate HUVEC adhesion molecule expression and adhesiveness. These results suggest that acidosis/GPR4 signaling regulates endothelial cell adhesion mainly through the G(s)/cAMP/Epac pathway and may play a role in the inflammatory response of vascular endothelial cells.
Conflict of interest statement
Figures
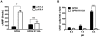


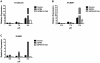
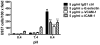
References
-
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. - PubMed
-
- Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–699. - PubMed
-
- Koul PB. Diabetic ketoacidosis: a current appraisal of pathophysiology and management. Clin Pediatr (Phila) 2009;48:135–144. - PubMed
-
- Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol. 2001;69:522–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

