I-MOVE multi-centre case control study 2010-11: overall and stratified estimates of influenza vaccine effectiveness in Europe
- PMID: 22110695
- PMCID: PMC3216983
- DOI: 10.1371/journal.pone.0027622
I-MOVE multi-centre case control study 2010-11: overall and stratified estimates of influenza vaccine effectiveness in Europe
Abstract
Background: In the third season of I-MOVE (Influenza Monitoring Vaccine Effectiveness in Europe), we undertook a multicentre case-control study based on sentinel practitioner surveillance networks in eight European Union (EU) member states to estimate 2010/11 influenza vaccine effectiveness (VE) against medically-attended influenza-like illness (ILI) laboratory-confirmed as influenza.
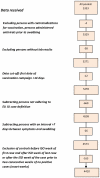
Methods: Using systematic sampling, practitioners swabbed ILI/ARI patients within seven days of symptom onset. We compared influenza-positive to influenza laboratory-negative patients among those meeting the EU ILI case definition. A valid vaccination corresponded to > 14 days between receiving a dose of vaccine and symptom onset. We used multiple imputation with chained equations to estimate missing values. Using logistic regression with study as fixed effect we calculated influenza VE adjusting for potential confounders. We estimated influenza VE overall, by influenza type, age group and among the target group for vaccination.
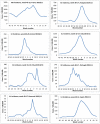
Results: We included 2019 cases and 2391 controls in the analysis. Adjusted VE was 52% (95% CI 30-67) overall (N = 4410), 55% (95% CI 29-72) against A(H1N1) and 50% (95% CI 14-71) against influenza B. Adjusted VE against all influenza subtypes was 66% (95% CI 15-86), 41% (95% CI -3-66) and 60% (95% CI 17-81) among those aged 0-14, 15-59 and ≥60 respectively. Among target groups for vaccination (N = 1004), VE was 56% (95% CI 34-71) overall, 59% (95% CI 32-75) against A(H1N1) and 63% (95% CI 31-81) against influenza B.
Conclusions: Results suggest moderate protection from 2010-11 trivalent influenza vaccines against medically-attended ILI laboratory-confirmed as influenza across Europe. Adjusted and stratified influenza VE estimates are possible with the large sample size of this multi-centre case-control. I-MOVE shows how a network can provide precise summary VE measures across Europe.
Conflict of interest statement
Figures

References
-
- European Commission. 2009. Proposal for a Council recommendation on seasonal influenza vaccination. 353/final/2. Available from: http://ec.europa.eu/health/ph_threats/com/Influenza/docs/seasonflu_rec20.... Accessed October 2011.
-
- Mereckiene J, Cotter S, D'Ancona F, Giambi C, Nicoll A, et al. Differences in national influenza vaccination policies across the European Union, Norway and Iceland 2008-2009. Euro Surveill. 2010;15 - PubMed
-
- Valenciano M, Ciancio B, Moren A. First steps in the design of a system to monitor vaccine effectiveness during seasonal and pandemic influenza in EU/EEA Member States. Euro Surveill. 2008;13:19015. - PubMed
-
- Kissling E, Valenciano M, Falcao J, Larrauri A, Widgren K, et al. “I-MOVE” towards monitoring seasonal and pandemic influenza vaccine effectiveness: lessons learnt from a pilot multi-centric case-control study in Europe, 2008-9. Euro Surveill. 2009;14 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

