Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus
- PMID: 22110700
- PMCID: PMC3215740
- DOI: 10.1371/journal.pone.0027628
Differential proliferation rhythm of neural progenitor and oligodendrocyte precursor cells in the young adult hippocampus
Abstract
Oligodendrocyte precursor cells (OPCs) are a unique type of glial cells that function as oligodendrocyte progenitors while constantly proliferating in the normal condition from rodents to humans. However, the functional roles they play in the adult brain are largely unknown. In this study, we focus on the manner of OPC proliferation in the hippocampus of the young adult mice. Here we report that there are oscillatory dynamics in OPC proliferation that differ from neurogenesis in the subgranular zone (SGZ); the former showed S-phase and M-phase peaks in the resting and active periods, respectively, while the latter only exhibited M-phase peak in the active period. There is coincidence between different modes of proliferation and expression of cyclin proteins that are crucial for cell cycle; cyclin D1 is expressed in OPCs, while cyclin D2 is observed in neural stem cells. Similar to neurogenesis, the proliferation of hippocampal OPCs was enhanced by voluntary exercise that leads to an increase in neuronal activity in the hippocampus. These data suggest an intriguing control of OPC proliferation in the hippocampus.
Conflict of interest statement
Figures

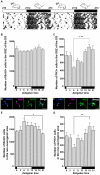

References
-
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. - PubMed
-
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. - PubMed
-
- Dawson MR, Polito A, Levine JM, Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. - PubMed
-
- Gensert JM, Goldman JE. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol. 2001;48:75–86. - PubMed
-
- Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

