Evaluation of the magnetic field requirements for nanomagnetic gene transfection
- PMID: 22110859
- PMCID: PMC3215215
- DOI: 10.3402/nano.v1i0.5167
Evaluation of the magnetic field requirements for nanomagnetic gene transfection
Abstract
The objective of this work was to examine the effects of magnet distance (and by proxy, field strength) on nanomagnetic transfection efficiency.
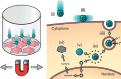
Methods: non-viral magnetic nanoparticle-based transfection was evaluated using both static and oscillating magnet arrays.
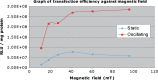
Results: Fluorescence intensity (firefly luciferase) of transfected H292 cells showed no increase using a 96-well NdFeB magnet array when the magnets were 5 mm from the cell culture plate or nearer. At 6 mm and higher, fluorescence intensity decreased systematically.
Conclusion: In all cases, fluorescence intensity was higher when using an oscillating array compared to a static array. For distances closer than 5 mm, the oscillating system also outperformed Lipofectamine 2000™.
Keywords: magnetic field; magnetic nanoparticle-based gene transfection; magnetic nanoparticles; non-viral gene delivery.
Figures


Similar articles
-
Oscillating Magnet Array-Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies.Nanomaterials (Basel). 2017 Jan 29;7(2):28. doi: 10.3390/nano7020028. Nanomaterials (Basel). 2017. PMID: 28336862 Free PMC article.
-
Oscillating magnet array-based nanomagnetic gene transfection of human mesenchymal stem cells.Nanomedicine (Lond). 2014 May;9(7):989-97. doi: 10.2217/nnm.13.74. Epub 2013 Jul 31. Nanomedicine (Lond). 2014. PMID: 23901783
-
Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts.Materials (Basel). 2013 Jan 18;6(1):255-264. doi: 10.3390/ma6010255. Materials (Basel). 2013. PMID: 28809306 Free PMC article.
-
A review of magnet systems for targeted drug delivery.J Control Release. 2019 May 28;302:90-104. doi: 10.1016/j.jconrel.2019.03.031. Epub 2019 Apr 1. J Control Release. 2019. PMID: 30946854 Review.
-
Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery.Gene Ther. 2006 Feb;13(4):283-7. doi: 10.1038/sj.gt.3302720. Gene Ther. 2006. PMID: 16462855 Review.
Cited by
-
Force-Mediating Magnetic Nanoparticles to Engineer Neuronal Cell Function.Front Neurosci. 2018 May 15;12:299. doi: 10.3389/fnins.2018.00299. eCollection 2018. Front Neurosci. 2018. PMID: 29867315 Free PMC article. Review.
-
Wireless neuromodulation in vitro and in vivo by intrinsic TRPC-mediated magnetomechanical stimulation.Commun Biol. 2022 Nov 2;5(1):1166. doi: 10.1038/s42003-022-04124-y. Commun Biol. 2022. PMID: 36323817 Free PMC article.
-
Enhanced nanomagnetic gene transfection of human prenatal cardiac progenitor cells and adult cardiomyocytes.PLoS One. 2013 Jul 31;8(7):e69812. doi: 10.1371/journal.pone.0069812. Print 2013. PLoS One. 2013. PMID: 23936108 Free PMC article.
-
Oscillating Magnet Array-Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies.Nanomaterials (Basel). 2017 Jan 29;7(2):28. doi: 10.3390/nano7020028. Nanomaterials (Basel). 2017. PMID: 28336862 Free PMC article.
-
Transfection types, methods and strategies: a technical review.PeerJ. 2021 Apr 21;9:e11165. doi: 10.7717/peerj.11165. eCollection 2021. PeerJ. 2021. PMID: 33976969 Free PMC article.
References
-
- Dobson J. Magnetic nanoparticle-based gene delivery. Gene Ther. 2006;13:283–7. - PubMed
-
- Plank C, Schillinger U, Scherer F, Bergemann C, Remy JS, Krotz F, et al. The magnetofection method: using magnetic force to enhance gene therapy. Biol Chem. 2003;384:737–47. - PubMed
-
- Mah C, Zolotukhin I, Fraites TJ, Dobson J, Batich C, Byrne BJ. Microsphere-mediated delivery of recombinant AAV vectors in vitro and in vivo. Molec Ther. 2000;1:S239.
-
- Mah C, Fraites TJ, Zolotukhin I, Song SH, Flotte TR, Dobson J, et al. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol Ther. 2002;6:106–12. - PubMed
-
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Kruger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and vivo. Gene Ther. 2002;2:102–9. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
