Daily or intermittent budesonide in preschool children with recurrent wheezing
- PMID: 22111718
- PMCID: PMC3247621
- DOI: 10.1056/NEJMoa1104647
Daily or intermittent budesonide in preschool children with recurrent wheezing
Abstract
Background: Daily inhaled glucocorticoids are recommended for young children at risk for asthma exacerbations, as indicated by a positive value on the modified asthma predictive index (API) and an exacerbation in the preceding year, but concern remains about daily adherence and effects on growth. We compared daily therapy with intermittent therapy.
Methods: We studied 278 children between the ages of 12 and 53 months who had positive values on the modified API, recurrent wheezing episodes, and at least one exacerbation in the previous year but a low degree of impairment. Children were randomly assigned to receive a budesonide inhalation suspension for 1 year as either an intermittent high-dose regimen (1 mg twice daily for 7 days, starting early during a predefined respiratory tract illness) or a daily low-dose regimen (0.5 mg nightly) with corresponding placebos. The primary outcome was the frequency of exacerbations requiring oral glucocorticoid therapy.
Results: The daily regimen of budesonide did not differ significantly from the intermittent regimen with respect to the frequency of exacerbations, with a rate per patient-year for the daily regimen of 0.97 (95% confidence interval [CI], 0.76 to 1.22) versus a rate of 0.95 (95% CI, 0.75 to 1.20) for the intermittent regimen (relative rate in the intermittent-regimen group, 0.99; 95% CI, 0.71 to 1.35; P=0.60). There were also no significant between-group differences in several other measures of asthma severity, including the time to the first exacerbation, or adverse events. The mean exposure to budesonide was 104 mg less with the intermittent regimen than with the daily regimen.
Conclusions: A daily low-dose regimen of budesonide was not superior to an intermittent high-dose regimen in reducing asthma exacerbations. Daily administration led to greater exposure to the drug at 1 year. (Funded by the National Heart, Lung, and Blood Institute and others; MIST ClinicalTrials.gov number, NCT00675584.).
Figures

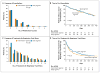
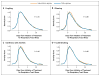
Comment in
-
Budesonide in preschool-age children with recurrent wheezing.N Engl J Med. 2012 Feb 9;366(6):570; author reply 571. doi: 10.1056/NEJMc1114826. N Engl J Med. 2012. PMID: 22316455 No abstract available.
-
Budesonide in preschool-age children with recurrent wheezing.N Engl J Med. 2012 Feb 9;366(6):570; author reply 571. doi: 10.1056/NEJMc1114826. N Engl J Med. 2012. PMID: 22316456 No abstract available.
-
Budesonide in preschool-age children with recurrent wheezing.N Engl J Med. 2012 Feb 9;366(6):570-1; author reply 571. doi: 10.1056/NEJMc1114826. N Engl J Med. 2012. PMID: 22316457 No abstract available.
-
Daily and intermittent corticosteroids have similar impact on recurrent wheezing in young children.J Pediatr. 2012 May;160(5):881. doi: 10.1016/j.jpeds.2012.01.060. J Pediatr. 2012. PMID: 22516330 No abstract available.
References
-
- Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–7. - PubMed
-
- Horner CC, Bacharier LB. Management approaches to intermittent wheezing in young children. Curr Opin Allergy Clin Immunol. 2007;7:180–4. - PubMed
-
- Bacharier LB, Phillips BR, Bloomberg GR, et al. Severe intermittent wheezing in preschool children: a distinct phenotype. J Allergy Clin Immunol. 2007;119:604–10. - PubMed
-
- Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma — United States, 1980–1999. MMWR Surveill Summ. 2002;51:1–13. - PubMed
-
- Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. J Asthma. 2005;42:373–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 5U10HL064305/HL/NHLBI NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- M01 RR00051/RR/NCRR NIH HHS/United States
- U10 HL064288/HL/NHLBI NIH HHS/United States
- UL1 RR025011/RR/NCRR NIH HHS/United States
- 5U10HL064313/HL/NHLBI NIH HHS/United States
- 5U10HL064288/HL/NHLBI NIH HHS/United States
- 5U10HL064295/HL/NHLBI NIH HHS/United States
- M01 RR000997/RR/NCRR NIH HHS/United States
- 5M01 RR00997/RR/NCRR NIH HHS/United States
- U10 HL064313/HL/NHLBI NIH HHS/United States
- UL1 TR000154/TR/NCATS NIH HHS/United States
- U10 HL064307/HL/NHLBI NIH HHS/United States
- M01 RR000036/RR/NCRR NIH HHS/United States
- U10 HL064295/HL/NHLBI NIH HHS/United States
- UL1 RR024992/RR/NCRR NIH HHS/United States
- U10 HL064287/HL/NHLBI NIH HHS/United States
- 5U10HL064307/HL/NHLBI NIH HHS/United States
- U10 HL064305/HL/NHLBI NIH HHS/United States
- 5UL1RR02499204/RR/NCRR NIH HHS/United States
- M01 RR000051/RR/NCRR NIH HHS/United States
- M01 RR00036/RR/NCRR NIH HHS/United States
- 1UL1RR025011/RR/NCRR NIH HHS/United States
- 5U10HL064287/HL/NHLBI NIH HHS/United States
- UL1RR025780/RR/NCRR NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
