A different immunologic profile characterizes patients with HER-2-overexpressing and HER-2-negative locally advanced breast cancer: implications for immune-based therapies
- PMID: 22112244
- PMCID: PMC3326559
- DOI: 10.1186/bcr3060
A different immunologic profile characterizes patients with HER-2-overexpressing and HER-2-negative locally advanced breast cancer: implications for immune-based therapies
Abstract
Introduction: The clinical efficacy of trastuzumab and taxanes is at least partly related to their ability to mediate or promote antitumor immune responses. On these grounds, a careful analysis of basal immune profile may be capital to dissect the heterogeneity of clinical responses to these drugs in patients with locally advanced breast cancer undergoing neoadjuvant chemotherapy.
Methods: Blood samples were collected from 61 locally advanced breast cancers (36 HER2- and 25 HER2+) at diagnosis and from 23 healthy women. Immunophenotypic profiling of circulating and intratumor immune cells, including regulatory T (Treg) cells, was assessed by flow cytometry and immunohistochemistry, respectively. Serum levels of 10 different cytokines were assessed by multiplex immunoassays. CD8+ T cell responses to multiple tumor-associated antigens (TAA) were evaluated by IFN-γ-enzyme-linked immunosorbent spot (ELISPOT). The Student's t test for two tailed distributions and the Wilcoxon two-sample test were used for the statistical analysis of the data.
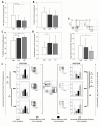
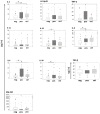
Results: The proportion of circulating immune effectors was similar in HER2+ patients and healthy donors, whereas higher percentages of natural killer and Treg cells and a lower CD4+/CD8+ T cell ratio (with a prevalence of naïve and central memory CD8+ T cells) were observed in HER2- cases. Higher numbers of circulating CD8+ T cells specific for several HLA-A*0201-restricted TAA-derived peptides were observed in HER2+ cases, together with a higher prevalence of intratumor CD8+ T cells. Serum cytokine profile of HER2+ patients was similar to that of controls, whereas HER2- cases showed significantly lower cytokine amounts compared to healthy women (IL-2, IL-8, IL-6) and HER2+ cases (IL-2, IL-1β, IL-8, IL-6, IL-10).
Conclusions: Compared to HER2- cases, patients with HER2-overexpressing locally advanced breast cancer show a more limited tumor-related immune suppression. This may account for the clinical benefit achieved in this subset of patients with the use of drugs acting through, but also promoting, immune-mediated effects.
Figures


References
-
- Borg A, Tandon AK, Sigurdsson H, Clark GM, Ferno M, Fuqua SA, Killander D, McGuire WL. HER-2/neu amplification predicts poor survival in node-positive breast cancer. Cancer Res. 1990;50:4332–4337. - PubMed
-
- Paik S, Hazan R, Fisher ER, Sass RE, Fisher B, Redmond C, Schlessinger J, Lippman ME, King CR. Pathologic findings from the National Surgical Adjuvant Breast and Bowel Project: prognostic significance of erbB-2 protein overexpression in primary breast cancer. J Clin Oncol. 1990;8:103–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

