Gliovascular and cytokine interactions modulate brain endothelial barrier in vitro
- PMID: 22112345
- PMCID: PMC3248576
- DOI: 10.1186/1742-2094-8-162
Gliovascular and cytokine interactions modulate brain endothelial barrier in vitro
Abstract
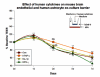
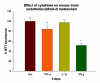
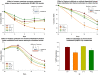
The glio-vascular unit (G-unit) plays a prominent role in maintaining homeostasis of the blood-brain barrier (BBB) and disturbances in cells forming this unit may seriously dysregulate BBB. The direct and indirect effects of cytokines on cellular components of the BBB are not yet unclear. The present study compares the effects of cytokines and cytokine-treated astrocytes on brain endothelial barrier. 3-dimensional transwell co-cultures of brain endothelium and related-barrier forming cells with astrocytes were used to investigate gliovascular barrier responses to cytokines during pathological stresses. Gliovascular barrier was measured using trans-endothelial electrical resistance (TEER), a sensitive index of in vitro barrier integrity. We found that neither TNF-α, IL-1β or IFN-γ directly reduced barrier in human or mouse brain endothelial cells or ECV-304 barrier (independent of cell viability/metabolism), but found that astrocyte exposure to cytokines in co-culture significantly reduced endothelial (and ECV-304) barrier. These results indicate that the barrier established by human and mouse brain endothelial cells (and other cells) may respond positively to cytokines alone, but that during pathological conditions, cytokines dysregulate the barrier forming cells indirectly through astrocyte activation involving reorganization of junctions, matrix, focal adhesion or release of barrier modulating factors (e.g. oxidants, MMPs).
© 2011 Chaitanya et al; licensee BioMed Central Ltd.
Figures



Similar articles
-
Effects of pericytes and various cytokines on integrity of endothelial monolayer originated from blood-nerve barrier: an in vitro study.J Med Dent Sci. 1999 Mar;46(1):31-40. J Med Dent Sci. 1999. PMID: 12160211
-
Effects of transient loss of shear stress on blood-brain barrier endothelium: role of nitric oxide and IL-6.Brain Res. 2003 Jul 11;977(2):239-46. doi: 10.1016/s0006-8993(03)02689-1. Brain Res. 2003. PMID: 12834884
-
Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells.Brain Res. 2007 May 25;1147:39-50. doi: 10.1016/j.brainres.2007.02.029. Epub 2007 Feb 24. Brain Res. 2007. PMID: 17368578 Free PMC article.
-
Astrocyte-endothelial interactions and blood-brain barrier permeability.J Anat. 2002 Jun;200(6):629-38. doi: 10.1046/j.1469-7580.2002.00064.x. J Anat. 2002. PMID: 12162730 Free PMC article. Review.
-
Brain endothelial cells and the glio-vascular complex.Cell Tissue Res. 2009 Jan;335(1):75-96. doi: 10.1007/s00441-008-0658-9. Epub 2008 Jul 16. Cell Tissue Res. 2009. PMID: 18633647 Review.
Cited by
-
Islet Amyloid Polypeptide: A Partner in Crime With Aβ in the Pathology of Alzheimer's Disease.Front Mol Neurosci. 2020 Mar 20;13:35. doi: 10.3389/fnmol.2020.00035. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32265649 Free PMC article. Review.
-
Annexin A2 Plus Low-Dose Tissue Plasminogen Activator Combination Attenuates Cerebrovascular Dysfunction After Focal Embolic Stroke of Rats.Transl Stroke Res. 2017 Dec;8(6):549-559. doi: 10.1007/s12975-017-0542-6. Epub 2017 Jun 4. Transl Stroke Res. 2017. PMID: 28580536
-
High-Fat Diet Increases Amylin Accumulation in the Hippocampus and Accelerates Brain Aging in hIAPP Transgenic Mice.Front Aging Neurosci. 2019 Aug 27;11:225. doi: 10.3389/fnagi.2019.00225. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31507407 Free PMC article.
-
Venous endothelial injury in central nervous system diseases.BMC Med. 2013 Oct 11;11:219. doi: 10.1186/1741-7015-11-219. BMC Med. 2013. PMID: 24228622 Free PMC article. Review.
-
Inhibition of Complement Drives Increase in Early Growth Response Proteins and Neuroprotection Mediated by Salidroside After Cerebral Ischemia.Inflammation. 2018 Mar;41(2):449-463. doi: 10.1007/s10753-017-0701-7. Inflammation. 2018. PMID: 29198014
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

