Mitigating preventable chronic disease: Progress report of the Cleveland Clinic's Lifestyle 180 program
- PMID: 22112436
- PMCID: PMC3264524
- DOI: 10.1186/1743-7075-8-83
Mitigating preventable chronic disease: Progress report of the Cleveland Clinic's Lifestyle 180 program
Abstract
Background: Poor lifestyle choices are key in development and progression of preventable chronic diseases. The purpose of the study was to design and test a program to mitigate the physical and fiscal consequences of chronic diseases.
Methods: Here we report the outcomes for 429 participants with one or more chronic conditions, including obesity, hypertension, hyperlipidemia and diabetes mellitus, many of whom had failed traditional disease management programs, who enrolled into a comprehensive lifestyle intervention. The Lifestyle 180 program integrates nutrition, physical activity and stress management interventions and was conducted at the Wellness Institute of the Cleveland Clinic, United States. An intensive 6 week immersion course, with 8 hours of group instruction per week, was followed by 3 follow-up, 4 hour-long sessions over the course of 6 months.
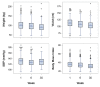
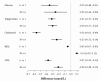
Results: Changes in biometric (weight, height, waist circumference, resting heart rate and blood pressure) and laboratory variables (fasting lipid panel, blood glucose, insulin, hemoglobin A1c, ultra sensitive C-reactive protein) at 6 months were compared with baseline (pre-post analysis). At week 30, biometric and laboratory data were available for 244 (57%) and 299 (70%) participants, respectively. These had a mean ± SD reduction in weight (6.8 ± 6.9 kg, P < 0.001), waist circumference (6.1 ± 7.3 cm, P < 0.001), glucose (4.5 ± 29.6 mg/dL or 0.25 ± 1.64 mmol/L, P = 0.009), triglycerides (26.4 ± 58.5 mg/dL or 0.30 ± 0.66 mmol/L, P < 0.001), low-density lipoprotein cholesterol (LDL) (7.9 ± 25.1 mg/dL or 0.2 ± 0.65 mmol/L, P < 0.001), hemoglobin A1c (HgbA1c) (0.20 ± 0.64%, P = 0.001), insulin (3.8 ± 11 microU/ml or 26.6 ± 76.4 ρmol, P < 0.001) and ultra sensitive C-reactive protein (US - CRP) (0.9 ± 4.8 mg/dL or 7.3 ± 40.2 nmol/L, P = 0.012), an increase in mean high-density lipoprotein cholesterol (HDL) (3.7 ± 8.4 mg/dL or 0.1 ± 0.22, P < 0.001), and decreased use of medications.
Conclusion: Implementation of a comprehensive lifestyle modification program among adults with common chronic conditions results in significant and clinically meaningful improvements in biometric and laboratory outcomes after 6 months.
Figures
References
-
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 916. 2003. - PubMed
-
- American College of Preventive Medicine: Lifestyle Medicine-Evidence Review. 2009. http://www.acpm.org/lifestyleMedicine.htm Accessed July 4, 2010.
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

