Sheathing the swords of death: post-translational modulation of plant metacaspases
- PMID: 22112449
- PMCID: PMC3337205
- DOI: 10.4161/psb.6.12.18247
Sheathing the swords of death: post-translational modulation of plant metacaspases
Abstract
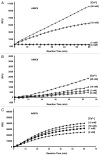
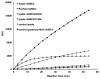
Plant metacaspases (MCPs) are conserved cysteine proteases that have been postulated as regulators of programmed cell death (PCD). Although MCPs have been proven to have PCD relevant functions in multiple species ranging from fungi to plants, how these proteases are modulated in vivo remains unclear. Aside from demonstrating that these proteases are distinct from metazoan caspases due to their different target site specificities, how these proteases are used to tightly regulate cell death progression is a key question that remains to be resolved. Some recent studies on the biochemical characteristics of type-II MCP activities in Arabidopsis may begin to shed additional light on this aspect. The in vitro catalytic activities of recombinant AtMC4, AtMC5 and AtMC8 are found to be Ca(2+)-dependent while recombinant AtMC9 is active under mildly acidic conditions and not dependent on stimulation by Ca(2+). Alterations of cellular pH and Ca(2+) concentration commonly occur during various stresses and may help to orchestrate differential activation of latent MCPs under these conditions. Recent peptide mapping for recombinant AtMC4 (also called Metacaspase-2d) followed by site-specific mutagenesis studies have revealed multiple potential self-cleavage sites with the identification of a conserved lysine residue (Lys-225) as the key position for enzyme function both in vitro and in vivo. The multiple self-cleavage sites in MCPs may also facilitate desensitization of these proteases and can provide a means for fine-tuning their proteolytic activities in order to achieve more sensitive control of downstream processes.
© 2011 Landes Bioscience
Figures
Comment on
- Watanabe N, Lam E. Calcium-dependent activation and autolysis of Arabidopsis metacaspase 2d. J Biol Chem. 2011;286 doi: 10.1074/jbc.M110.194340. doi: 10.1074/jbc.M110.194340
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
