Desensitization and trafficking of μ-opioid receptors in locus ceruleus neurons: modulation by kinases
- PMID: 22113080
- PMCID: PMC3286302
- DOI: 10.1124/mol.111.076208
Desensitization and trafficking of μ-opioid receptors in locus ceruleus neurons: modulation by kinases
Abstract
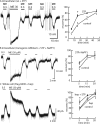
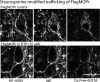
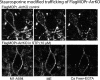
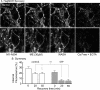
The phosphorylation of μ-opioid receptors (MOPRs) by G protein-coupled receptor kinases (GRKs), followed by arrestin binding, is thought to be a key pathway leading to desensitization and internalization. The present study used the combination of intracellular and whole-cell recordings from rats and mice, as well as live cell imaging of Flag-tagged MOPRs from mouse locus ceruleus neurons, to examine the role of protein kinases in acute desensitization and receptor trafficking. Inhibition of GRKs by using heparin or GRK2-mutant mice did not block desensitization or alter the rate of recovery from desensitization. The nonselective kinase inhibitor staurosporine did not reduce the extent of [Met(5)]enkephalin (ME)-induced desensitization but increased the rate of recovery from desensitization. In the presence of staurosporine, ME-activated FlagMOPRs were internalized but did not traffic away from the plasma membrane. The increased rate of recovery from desensitization correlated with the enhancement in the recycling of receptors to the plasma membrane. ME-induced MOPR desensitization persisted and the trafficking of receptors was modified after inhibition of protein kinases. The results suggest that desensitization of MOPRs may be an early step after agonist binding that is modulated by but is not dependent on kinase activity.
Figures


References
-
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. (2006) Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell 127:85–97 - PubMed
-
- Bailey CP, Kelly E, Henderson G. (2004) Protein kinase C activation enhances morphine-induced rapid desensitization of μ-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol 66:1592–1598 - PubMed
-
- Benovic JL, Stone WC, Caron MG, Lefkowitz RJ. (1989) Inhibition of the β-adrenergic receptor kinase by polyanions. J Biol Chem 264:6707–6710 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

