Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension
- PMID: 22113951
- PMCID: PMC3415620
- DOI: 10.1002/jbmr.1479
Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension
Abstract
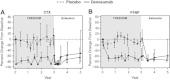
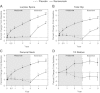
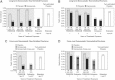
The 3-year FREEDOM trial assessed the efficacy and safety of 60 mg denosumab every 6 months for the treatment of postmenopausal women with osteoporosis. Participants who completed the FREEDOM trial were eligible to enter an extension to continue the evaluation of denosumab efficacy and safety for up to 10 years. For the extension results presented here, women from the FREEDOM denosumab group had 2 more years of denosumab treatment (long-term group) and those from the FREEDOM placebo group had 2 years of denosumab exposure (cross-over group). We report results for bone turnover markers (BTMs), bone mineral density (BMD), fracture rates, and safety. A total of 4550 women enrolled in the extension (2343 long-term; 2207 cross-over). Reductions in BTMs were maintained (long-term group) or occurred rapidly (cross-over group) following denosumab administration. In the long-term group, lumbar spine and total hip BMD increased further, resulting in 5-year gains of 13.7% and 7.0%, respectively. In the cross-over group, BMD increased at the lumbar spine (7.7%) and total hip (4.0%) during the 2-year denosumab treatment. Yearly fracture incidences for both groups were below rates observed in the FREEDOM placebo group and below rates projected for a "virtual untreated twin" cohort. Adverse events did not increase with long-term denosumab administration. Two adverse events in the cross-over group were adjudicated as consistent with osteonecrosis of the jaw. Five-year denosumab treatment of women with postmenopausal osteoporosis maintained BTM reduction and increased BMD, and was associated with low fracture rates and a favorable risk/benefit profile.
Trial registration: ClinicalTrials.gov NCT00523341.
© 2012 American Society for Bone and Mineral Research
Figures

References
-
- Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–25. - PubMed
-
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–42. - PubMed
-
- Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, Delmas P, Zoog HB, Austin M, Wang A, Kutilek S, Adami S, Zanchetta J, Libanati C, Siddhanti S, Christiansen C. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–65. - PubMed
-
- Eastell R, Christiansen C, Grauer A, Kutilek S, Libanati C, McClung MR, Reid IR, Resch H, Siris E, Uebelhart D, Wang A, Weryha G, Cummings SR. Effects of denosumab on bone turnover markers in postmenopausal osteoporosis. J Bone Miner Res. 2011;3:530–7. - PubMed
-
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–48. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

