Alcohol reduces airway hyperresponsiveness (AHR) and allergic airway inflammation in mice
- PMID: 22114149
- PMCID: PMC3289271
- DOI: 10.1152/ajplung.00077.2011
Alcohol reduces airway hyperresponsiveness (AHR) and allergic airway inflammation in mice
Abstract
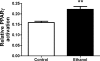
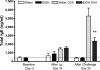
There is very limited knowledge about the effects of alcohol on airway hyperresponsiveness and inflammation in asthma. Historical accounts of alcohol administration to patients with breathing problems suggest that alcohol may have bronchodilating properties. We hypothesized that alcohol exposure will alter airway hyperresponsiveness (AHR) and pulmonary inflammation in a mouse model of allergic asthma. To test this hypothesis, BALB/c mice were fed either 18% alcohol or water and then sensitized and challenged with ovalbumin (OVA). AHR was assessed by means of ventilation or barometric plethysmography and reported as either total lung resistance or enhanced pause, respectively. Airway inflammation was assessed by total and differential cell counts in bronchoalveolar lavage fluid (BALF), cytokine levels in BALF, lung histology, and serum immunoglobulin E (IgE) levels. Alcohol feeding significantly blocked methacholine-induced increases in AHR compared with water-fed controls. Alcohol feeding significantly reduced total cell numbers (64%) as well as the number of eosinophils (84%) recruited to the lungs of these mice. Modest changes in lung pathology were also observed. Alcohol exposure led to a reduction of IgE in the serum of the EtOH OVA mice. These data demonstrate that alcohol exposure blunts AHR and dampens allergic airway inflammation indices in allergic mice and suggest that there may be an important role for alcohol in the modulation of asthma. These data provide an in vivo basis for previous clinical observations in humans substantiating the bronchodilator properties of alcohol and for the first time demonstrates an alcohol-induced reduction of allergic inflammatory cells in a mouse model of allergic asthma.
Figures
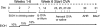





References
-
- Ballenger JJ. Experimental effect of cigarette smoke on human respiratory cilia. N Engl J Med 263: 832–835, 1960 - PubMed
-
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis 184: 1134–1142, 2001 - PubMed
-
- Bousquet J, Chanez P, Lacoste JY, Barneon G, Ghavanian N, Enander I, Venge P, Ahlstedt S, Simony-Lafontaine J, Godard P. Eosinophilic inflammation in asthma. N Engl J Med 323: 1033–1039, 1990 - PubMed
-
- Brown EA. The use of intravenous ethyl alcohol in the treatment of status asthmaticus. Ann Allergy 5: 193–195, 1947 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

