Hypoxia-inducible factor-1α regulates KCNMB1 expression in human pulmonary artery smooth muscle cells
- PMID: 22114151
- PMCID: PMC3289270
- DOI: 10.1152/ajplung.00302.2011
Hypoxia-inducible factor-1α regulates KCNMB1 expression in human pulmonary artery smooth muscle cells
Abstract
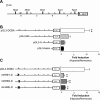
Previously, we observed that hypoxia increases the expression of the β1-subunit (KCNMB1) of the calcium-sensitive potassium channel (BK(Ca)). Herein, we elucidate the mechanism whereby hypoxia increases KCNMB1 expression in human pulmonary artery smooth muscle cells (hPASMC). In response to hypoxia, the expression of both the transcription factor hypoxia-inducible factor 1-α (HIF-1α) and KCNMB1 are increased. Knockdown of HIF-1α using a shRNA plasmid blocked the hypoxic induction of KCNMB1 expression. Chromatin immunoprecipitation (ChIP) demonstrated HIF-1α binding to three discrete regions of the human KCNMB1 promoter known to contain hypoxia response elements (HREs). A KCNMB1 promoter reporter assay combined with site-directed mutagenesis identified two adjacent HREs located between -3,540 bp and -3,311 bp that are essential for the hypoxic induction of KCNMB1 promoter activity. Furthermore, additional ChIP assays demonstrated recruitment of the HIF-1α transcriptional coactivator, p300, to this same promoter region. Treatment of hPASMC with the histone deacetylase inhibitor, trichostatin, prolonged the increase in KCNMB1 observed with hypoxia, suggesting that alterations in chromatin remodeling function to limit the hypoxic induction of KCNMB1. Finally, KCNMB1 knockdown potentiated the hypoxia-induced increase in cytosolic calcium in hPASMC, highlighting the contribution of the β1-subunit in modulating vascular SMC tone in response to acute hypoxia. In conclusion, HIF-1α increases KCNMB1 expression in response to hypoxia in hPASMC by binding to two HREs located at -3,540 to -3,311 of the KCNMB1 promoter. We speculate that selective modulation of KCNMB1 expression may serve as a novel therapeutic approach to address diseases characterized by an increase in vascular tone.
Figures





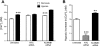
References
-
- Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res 93: 965–971, 2003 - PubMed
-
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science 256: 534–535, 1992 - PubMed
-
- Brenner R, Peréz GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Patterson AJ, Nelson MT, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 407: 870–876, 2000 - PubMed
-
- Cornfield DN, Saqueton CB, Porter VA, Herron JM, Resnik ER, Haddad IY, Reeve HL. Voltage-gated K+ channel activity in the ovine pulmonary vasculature is developmentally regulated. Am J Physiol Lung Cell Mol Physiol 278: L1297–L1304, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

